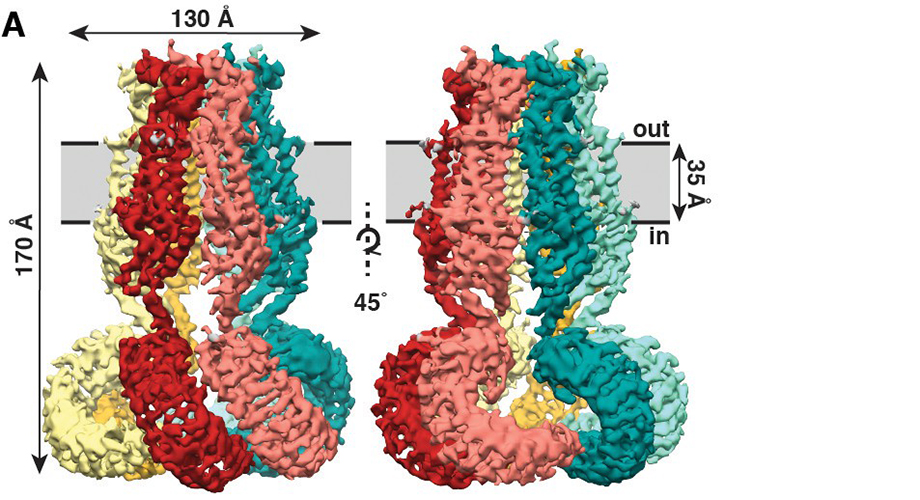
Overall architecture of homomeric SWELL1. (A) Cryo-EM reconstruction of SWELL1 homohexamer viewed from the membrane plane highlighting a dimer pair (top left, red and pink subunits) and an interface between dimers (top right, pink and green subunits), from the extracellular side (bottom left), and from the cytosolic side (bottom right). (B) Detailed view of SWELL1 ‘inner’ protomer. (C) Topology diagram denoting secondary structural elements. Dashed lines indicate unresolved regions on both protomers in a dimer pair, while dashed shape borders indicate regions that are only resolved on one protomer. (Kefauver et al.)
How a 'jellyfish'-shaped structure relieves pressure in your cells
Scripps Research team takes closer look at a mysterious protein.
August 16, 2018
Scientists at Scripps Research have solved the structure of a key protein that senses when our cells swell. This protein, called SWELL1 (or LRRC8A), works as an “ion channel” on the cell membrane to relieve pressure inside cells.
The new study, published recently in the journal eLIFE, shows that SWELL1 is made of six subunits that meet on top, bundling together to form a jellyfish-like mantle with six tendrils hanging down.
“This structure provides a first glimpse into how this ion channel senses changes in volume in a cell,” says Jennifer Kefauver, graduate student at Scripps Research, the Howard Hughes Medical Institute, and first author of the new study.
SWELL1 was discovered in 2014 in the lab of Ardem Patapoutian, PhD, professor at Scripps Research and investigator with the Howard Hughes Medical Institute. The discovery opened the door to crucial studies into how the protein functions.
The next important step was to shed light on SWELL1’s molecular structure. The scientists aimed to understand the basics of how this ion channel senses changes in volume. To do this, they needed to take a look at the channel’s molecular machinery.
Kefauver spearheaded studies as a joint student between the labs of Patapoutian and Andrew Ward, PhD, professor at Scripps Research and leader in a high-resolution imaging technique called cryo-electron microscopy (cryo-EM). Kefauver used cryo-EM techniques to solve SWELL1’s jellyfish-like structure and get a first look at how ions can travel through the channel’s central pore.
“Jennifer pursued the structure of SWELL1 with great tenacity, leaving no stone unturned and overcoming enumerable hurdles. It was wonderful to see the fruits of her labor result in such a beautiful structure,” says Ward.
This new look at the ion channel suggests that interacting parts of the tendrils—sites that have a positive or a negative charge—sense a change in ionic strength in the cell (a dilution of the cell’s salt contents as it absorbs water). The charged residues could send a signal up to the pore of the channel, telling the channel to release chloride ions from the cell and relieve the pressure.
Kefauver is hoping this new view of the structure can fuel medical research. SWELL1 has a role in at least one disease—an immune deficiency called agammaglobulinemia. “Having the structure is really important for scientists trying to understand how this channel works and what disease-causing mutations might do.”
Next, the researchers took a closer look at how the different parts of the SWELL1 structure affect channel function. They found that mutating the protein at either of two sites keeps the structure from properly controlling traffic through the ion channel.
The new study shows bundles of the SWELL1 subunit alone—and scientists know that SWELL1 has to be there for a functional channel. But up to four other subunits can swap into the structure at different sites. Kefauver says the next step is to determine how different combinations of SWELL1 subunits come together to form ion channels with different activities.
Additional authors of the study, “Structure of the human volume regulated anion channel,” include Kei Saotome, Adrienne E. Dubin, Stuart M. Cahalan, Gunhee Hong, and Tess Whitman of the Howard Hughes Medical Institute and Scripps Research; Jesper Pallesen, Christopher Cottrell and Wen-Hsin Lee of Scripps Research; Christopher Crowley of the Department of Dermatology, UC San Diego and Scripps Research; and Zhaozhu Qiu of the Genomics Institute of the Novartis Research Foundation.
The study was supported by the National Institutes of Health’s National Research Service Award (grant F31 NS093778-3), the National Institutes of Health (grant NS083174) and a Ray Thomas Edwards Foundation grant.
About Scripps Research
Scripps Research is ranked the most influential scientific institution in the world for its impact on innovation. A nonprofit research organization, Scripps expands basic knowledge in the biosciences and uses these fundamental advancements to develop profound innovations that improve well-being. Scripps researchers lead breakthrough studies that address the world’s most pressing health concerns, accelerating the creation and delivery of medical breakthroughs to better human health across the globe. Our educational and training programs mold talented and committed students and postdocs into the next generation of leading scientists.
For more information about Scripps Research visit www.scripps.edu. Follow @ScrippsResearch on Twitter, Facebook or LinkedIn.
Media Contact
Madeline McCurry-Schmidt, Scripps Research, madms@scripps.edu, 858-784-9254
For more information, contact press@scripps.edu

