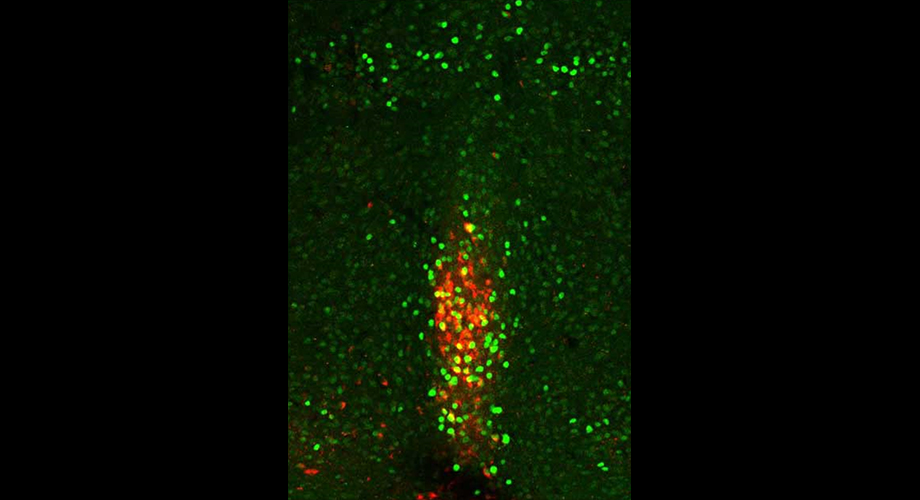
Neurons in the Xiphoid nucleus are activated by cold (green). A subset of this population (red) promotes the animal to eat more in the cold. Credit: Scripps Research
How cold temperatures trigger the brain to boost appetite
Scripps Research scientists’ discovery could lead to new weight loss and metabolic health treatments.
August 16, 2023
LA JOLLA, CA—Neuroscientists at Scripps Research have identified brain circuits that make mammals want to eat more when they are exposed to cold temperatures.
Mammals automatically burn more energy to maintain normal body temperature when exposed to cold. This cold-activated increase in energy expenditure triggers an increase in appetite and feeding, although the specific mechanism controlling this had been unknown. In the new study, reported on August 16, 2023, in Nature, the researchers identified a cluster of neurons that work as a “switch” for this cold-related, food-seeking behavior in mice. The discovery could lead to potential therapeutics for metabolic health and weight loss.
“This is a fundamental adaptive mechanism in mammals and targeting it with future treatments might allow the enhancement of the metabolic benefits of cold or other forms of fat burning,” says study senior author Li Ye, PhD, associate professor and the Abide-Vividion Chair in Chemistry and Chemical Biology at Scripps Research.
The study’s first author was Ye Lab postdoctoral research associate Neeraj Lal, PhD.
Because exposure to cold leads to enhanced energy burning to stay warm, cold water immersion and other forms of “cold therapy” have been explored as methods for losing weight and improving metabolic health. One drawback of cold therapies is that humans’ evolved responses to cold are not designed to cause weight loss (an effect that could have been fatal during the frequent periods of food scarcity in pre-modern times). Cold, like dieting and exercise, increases appetite to counteract any weight-loss effect. In the study, Ye and his team set out to identify the brain circuitry that mediates this cold-induced appetite increase.
One of their first observations was that, with the onset of cold temperatures (from 73F to 39F), mice increase their food seeking only after a delay of about six hours, suggesting this behavioral change is not simply a direct result of cold sensing.
Using techniques called whole-brain clearing and light sheet microscopy, the researchers compared the activity of neurons across the brain during cold versus warm conditions. Soon they made a key observation: While most of the neuronal activity across the brain was much lower in the cold condition, portions of a region called the thalamus showed higher activation.
Eventually, the team zeroed in on a specific cluster of neurons called the xiphoid nucleus of the midline thalamus, showing that activity in these neurons spiked under cold conditions just before the mice stirred from their cold-induced torpor to look for food. When less food was available at the onset of the cold condition, the activity increase in the xiphoid nucleus was even greater—suggesting that these neurons respond to a cold-induced energy deficit rather than cold itself.
When the researchers artificially activated these neurons, the mice increased their food-seeking, but not other activities. Similarly, when the team inhibited the activity of these neurons, the mice decreased their food-seeking. These effects appeared only under the cold condition, implying that cold temperatures provide a separate signal that must also be present for appetite changes to occur.
In a last set of experiments, the team showed that these xiphoid nucleus neurons project to a brain region called the nucleus accumbens—an area long known for its role in integrating reward and aversion signals to guide behavior, including feeding behavior.
Ultimately, these results may have clinical relevance, Ye says, for they suggest the possibility of blocking the usual cold-induced appetite increase, allowing relatively simple cold exposure regimens to drive weight loss much more efficiently.
“One of our key goals now is to figure out how to decouple the appetite increase from the energy-expenditure increase,” he says. “We also want to find out if this cold-induced appetite-increase mechanism is part of a broader mechanism the body uses to compensate for extra energy expenditure, for example after exercise.”
“Xiphoid nucleus of the midline thalamus controls cold-induced food seeking” was co-authored by Neeraj Lal, Samarth Aggarwal, Alan Zhang, Kristina Wang, Tianbo Qi, Zhengyuan Pang, Dong Yang, Victoria Nudell, and Li Ye, all of Scripps Research; Phuong Le and Gene Yeo of the University of California—San Diego; and Alexander Banks of Beth Israel Deaconess Medical Center.
Funding was provided by the National Institutes of Health (DP2DK128800, DK114165, DK124731, DK134609, MH132570), the Dana Foundation, the Whitehall Foundation, the Baxter Foundation and the Abide-Vividion Endowment.
For more information, contact press@scripps.edu

