Structure, Dynamics and Interactions of Proteins
Research overview
My laboratory utilizes high-resolution nuclear magnetic resonance (NMR) spectroscopy and other biophysical and biochemical methods to investigate the structure, dynamics, and folding mechanisms of proteins and to map their functional interactions. NMR is unique as a method for determining three-dimensional structures of proteins and protein complexes in solution and also providing novel information about the time-dependent structural fluctuations that are essential for protein function.
Our research interests include:
• Intrinsically disordered proteins and cellular signaling.
• Mechanisms of nucleic acid recognition by zinc fingers.
• Mechanisms of protein folding and misfolding.
• Protein dynamics and “invisible” excited states.
Image Gallery
Read more: Nature Reviews Molecular Cell Biology
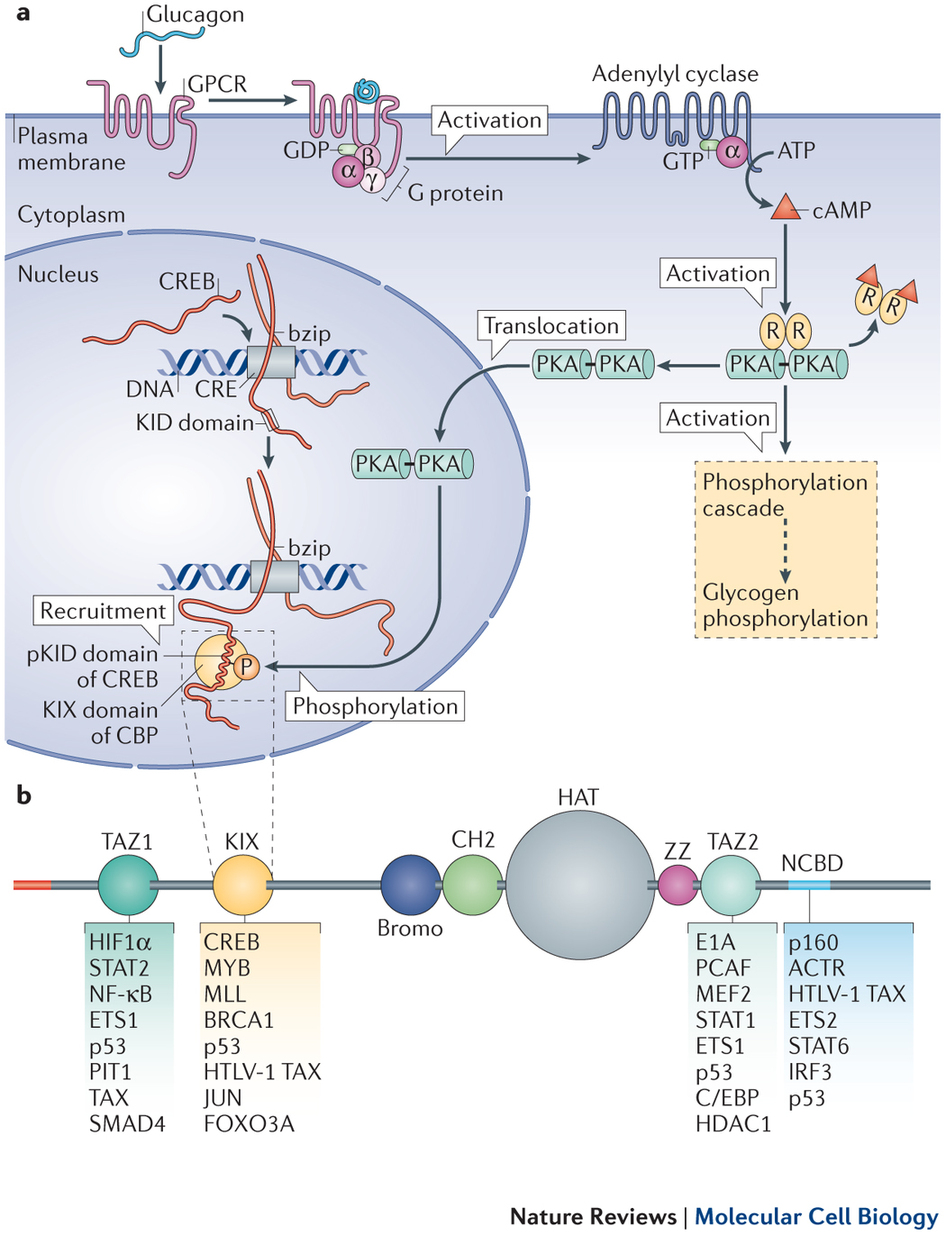
Intrinsically disordered proteins in cellular signalling and regulation.
Read more: PNAS
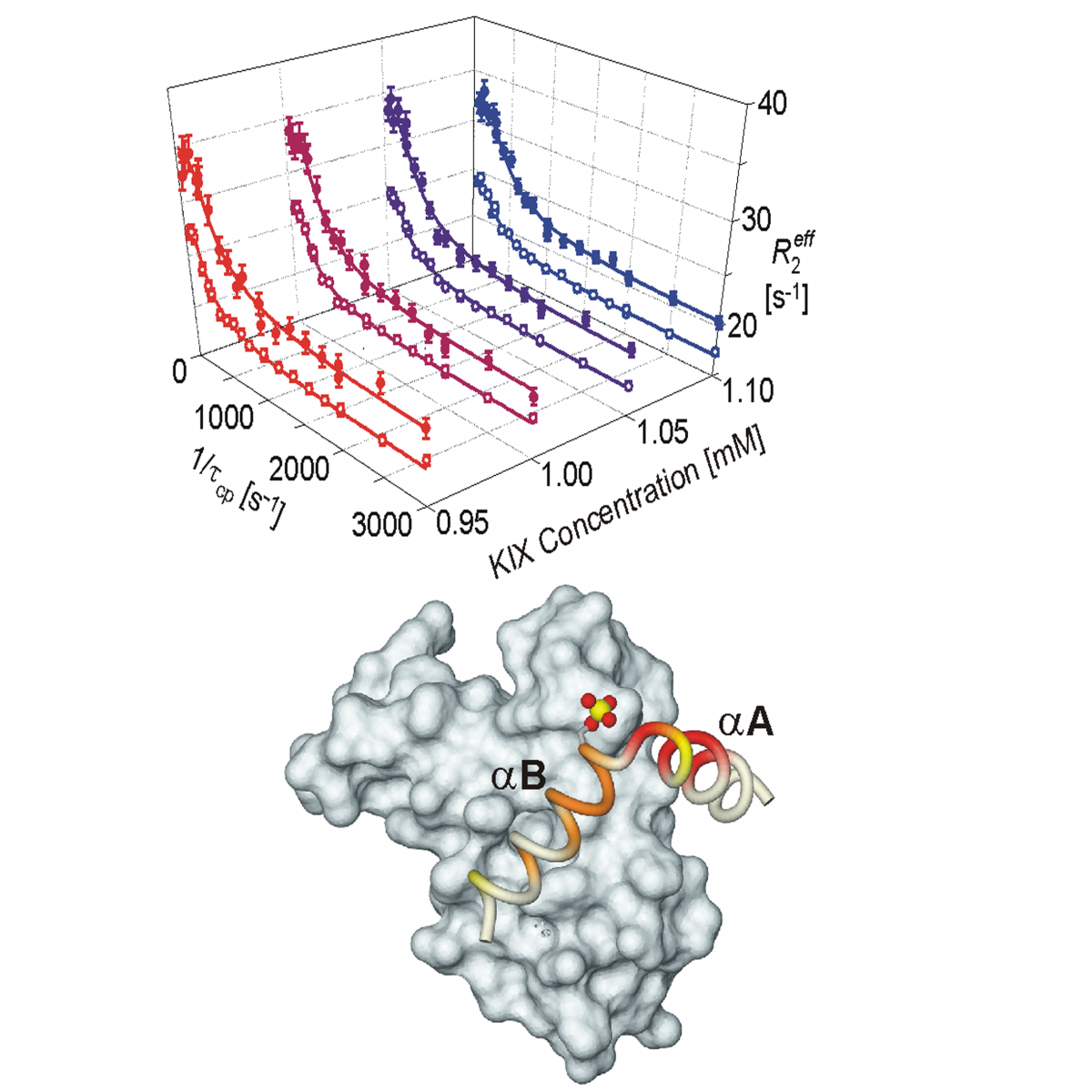
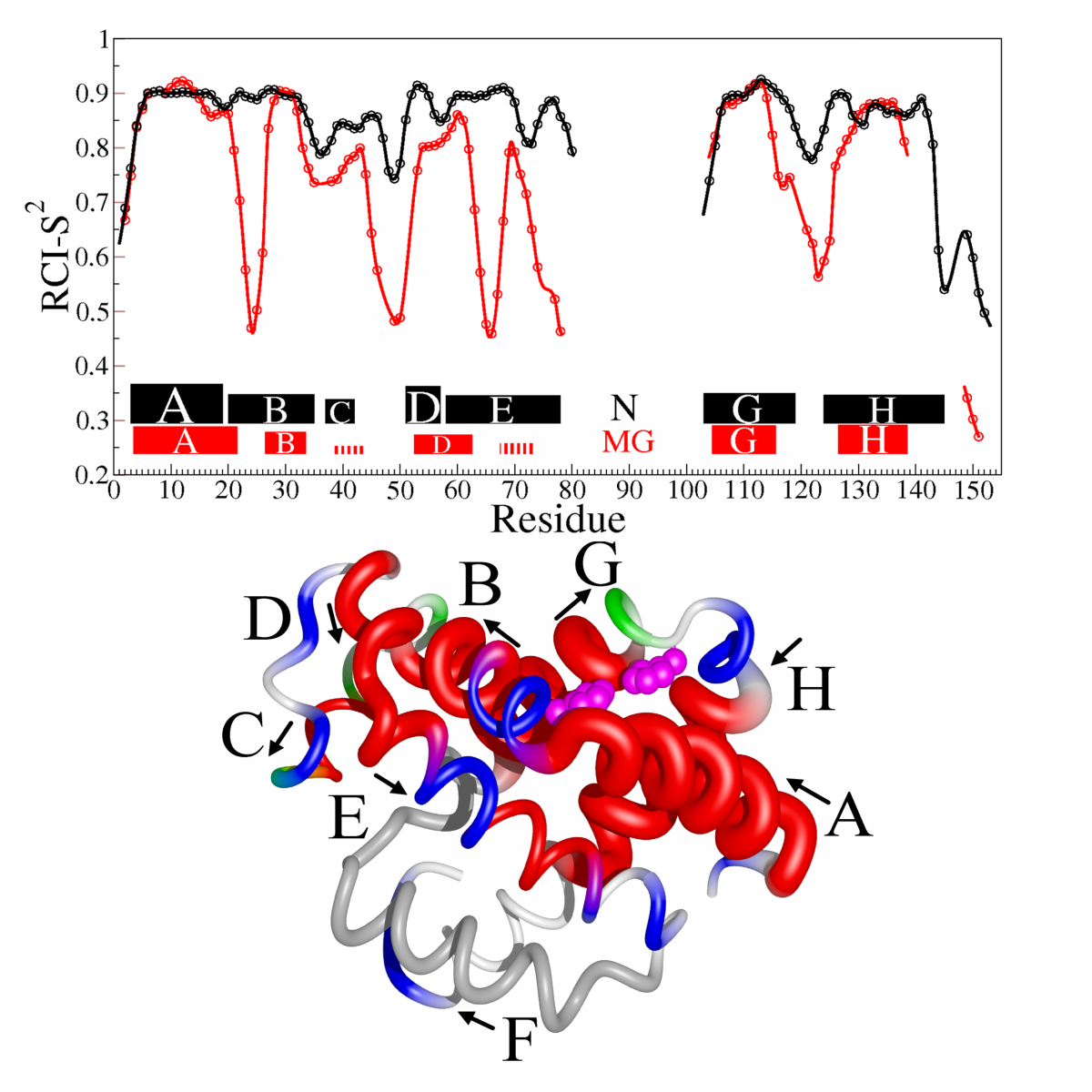
Measurement of protein unfolding/refolding kinetics and structural characterization of hidden intermediates by NMR relaxation dispersion.
Read more: PNAS
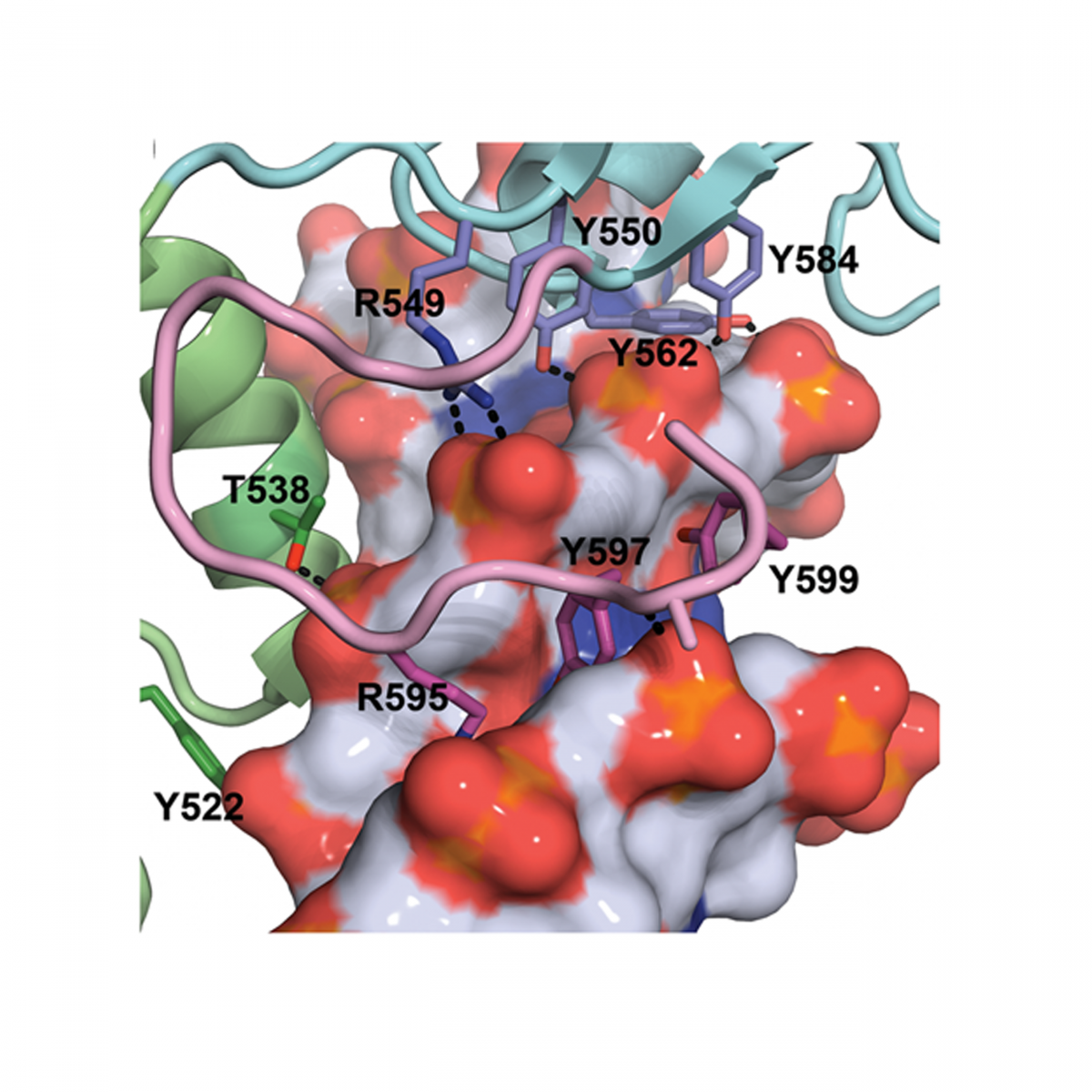
Molecular basis for recognition of methylated and specific DNA sequences by the zinc finger protein Kaiso.