Major Advances Towards the Four Grand ChallengesDefining the problem: Four Grand ChallengesCatalytic C–H activation reactions possess the transformative power that enables highly efficient synthesis of molecules, and hence sustainable chemical manufacturing. Although the capabilities of C–H activation have been recognized for nearly a century in chemical synthesis, fundamental challenges pertinent to the underlying chemistry must be overcome to truly harness its potential. It is essential to divide C–H functionalization (to which metal-catalyzed C–H activation reactions belong) into two subfields: first functionalization of alkane, mainly methane oxidation for fuel transportation or alkane functionalization for bulk chemical production; and further functionalization of synthetically relevant substrates, for making complex molecules with new functions.
The measurement for success in these the two subfields are different and the approaches for solving these two problems are fundamentally different. For the first functionalization, one extremely efficient reaction of one particular C–H bond on an unfunctionalized substrate is needed. In contrast, to realize the full potential of the further C–H functionalization for synthesis, four fundamental challenges – the Y’s of C–H activation – must be addressed:
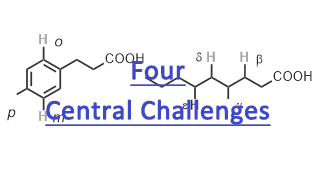
ReactivitY: Developing diverse carbon-carbon and carbon-heteroatom
bond forming reactions of a range of poorly reactive native substrates; Despite century-long efforts on understanding and developing C–H activation reactions, seeking solutions to these problems has met with limited success due to: lack of reactivity of broadly useful substrates; lack of well-defined stereomodel for enantioselective metal insertion; lack of predictable approaches for controlling site selectivity as majority of C–H bonds are electronically and sterically similar, hence difficult to distinguish; and most importantly, lack of ligands that can accelerate C–H activation reactions.
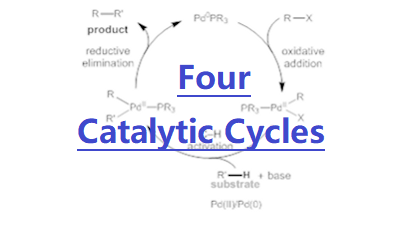
Addressing the four challenges: New concepts for C−H activation and catalyst design
1. Weakly coordinating substrates which recruit and position the Pd catalyst for C–H activation
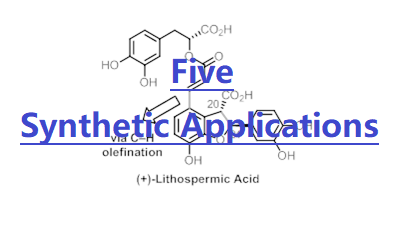
whilst avoiding the scope limitations associated with strongly binding directing groups. These approaches and concepts have been widely adopted – as recently reported by Chemistry World, our lab (Prof. Yu as author) is the most widely cited in the field of C–H activation. We project these insights in the still-nascent field could potentially enable more general, modular, and diverse C-H activation reactions in the future. Applications and Impact:1. Yu group's reaction and catalyst development has led to substantial collaborations with more than 20 research groups in the community: Blackmond, Baran, Kelly, Cravatt, Berry, Houk, Musaev, Morokuma, Sigman, Jones, Bertrand, Romero, Neidig, Sorensen, Stoltz, Davies, Movassaghi, Sarpong, Sunoj, Marder, Ye, Lei. 2. More than 50 other laboratories have successfully used our bifunctional MPAA ligand for developing C-H activation reactions as described in 121 publications. 3. Fürstner, Movassaghi, Davies, Kumamoto, Sorensen, Sarpong, X. Lei, A. Li, D. Mal, M. Cushman, B. F. Shi, A. Ghosh, A. B. Smith, and R. A. Barrow, among others, have successfully applied our C-H activation reactions in their total syntheses natural products. 4. The pharmaceutical and agricultural industries, specifically BMS, VRTX, Pfizer, Eisai, Abide, Abbvie, Boehringer-Ingelheim, Novartis, and Syngenta have used his C–H activation methods in their drug discovery efforts as indicated by the collaborative publications. For example, one of our C–H lactonization reactions has been used by Pfizer to create potent positive allosteric modulators (PAM) that are extremely challenging (J. Med. Chem. 2017, 60, 6649). One of our C–H arylation reactions has recently been employed by a process team at Novartis as the superior route (Org. Lett. 2016, 18, 3310), halving step count and improving the overall yield by 17-fold. 5. Yu’s technologies have also benefited two successful drug discovery biotechs. His enantioselective C–H methylation reaction of pyrrolidine has led to a drug candidate at Abide Therapeuticals that is recently acquired by Lundbeck for $400 millions. His C–H arylation of free alkyl amines, and C–H coupling of phenyl acetic acids have been used by Vividion Therapeutics (Yu as a co-founder) to synthesize a designed library for drug discovery. This company with many promising drug candidates has been recently acquired by Bayer for $2 billion. 6. Yu’s approaches and concepts have been widely adopted – as recently reported by Chemistry World, he is the most widely cited in the field of C–H activation with annual citation of 4000–4500 on C-H activation. Future Research Directions:We aim to realize the activation of C(sp3)–H bonds that are one more carbon away from the native functional groups, for example, achieving both γ and δ-C–H activation reactions of free alcohols, including in enantioselective transformations (reactivity and enantioselecivity). We will continue to develop superior catalysts for non-directed C-H activation reactions of arenes that will complement the directed approach. We seek to build upon our very recent discoveries in biomimetic C–H hydroxylations to develop new C(sp3)–H hydroxylations which use molecular oxygen as the sole oxidant and oxygen source (practicality), harnessing our newly-developed next generation pyridine-pyridone bifunctional ligands. We also aim to develop new copper catalysts for C(sp3)–H activation reactions, given Cu's abundance and relative affordability (practicality). These transformations will both serve as a complementary approach to our efforts with Pd and continue our long-standing studies in Cu catalysis. Talks and Interviews:[1] 2013: 43rd National Organic Chemistry Symposium: Recent perspective and introduction of our research program. [Link] [2] 2014: Accelerated C-H Activation Reactions: A Shortcut to Molecular Complexity from Chemical Feedstock. [Link] [3] 2016: An interview by the MacArthur Foundation. [Link] [4] 2020: An interview by World Chemistry. Based on their analysis on multiple databases, we are the most cited laboratory in the area of C-H activation. [Link] [5] 2021: A recent interview by National Science Review discussing our perspectives about challenges and solutions in C–H activation chemistry. [Link] [6] 2022: A recent commentary on Jin about C–H activation for drug discovery: [Link] [7] 2023: BMS Symposium – 20-Year Dancing with Palladium and C–H bonds: From Curiosity to Industrialization. [Link] [8] Historic scientific events of the group. [Link] To learn more, please click the buttons on the right. |
|
|
|
|
Scripps Research Institute,
10550 North Torrey Pines Road La Jolla, CA 92037 Beckman 372 |

|