The Kelly Group Research
Parkinson's disease:
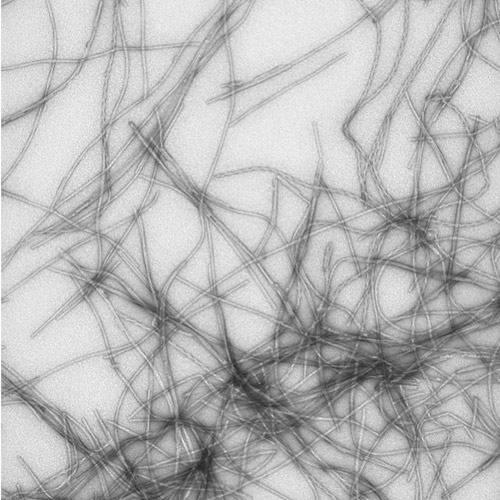
Figure 1: α-Synuclein is a neuronal protein implicated in several neurodegenerative diseases, including Parkinson's disease. Genetic factors account for approximately 10% of Parkinson's disease cases, and therefore it is important to identify sporadic (non-genetic) factors associated with the disease. We are currently investigating the effect of metabolites generated during oxidative stress on α-synuclein misfolding and aggregation. α-Synuclein aggregation is monitored in vitro using circular dichroism spectroscopy, thioflavin T fluorescence, size-exclusion chromatography, and electron microscopy. The figure shows an electron microscopy image of α-synuclein fibrils formed in the presence of the oxidative metabolite ketoaldehyde. Courtesy of Dr. Daryl Bosco.
The α-synucleinopathies, including Parkinson's disease and Dementia with Lewy Bodies, are characterized by the presence of cytoplasmic aggregates within degenerating dopaminergic neurons in the substantia nigra. These so called Lewy Bodies are mainly composed of 14.5 kDa α-synuclein fibrils and other aggregated proteins. Although some mutations in α-synuclein are correlated with Parkinson's disease, most cases seem to be sporadic. The sporadic cases appear to originate from oxidative stress. The possible correlation between oxidative stress/inflammation and Parkinson's disease is supported by Parkinson's disease animal models and genetics, the correlation between particular environmental neurotoxins that lead to an accumulation of reactive oxygen species and Parkinson's disease, and the sensitivity of dopaminergic neurons towards oxidative stress generated by dopamine metabolism. The main aim of this research project is to identify the role of reactive oxygen species in the α-synucleinopathies. It is feasible that reactive oxygen species react with metabolites generating oxidized metabolites that can interact with α-synuclein thereby triggering misfolding and subsequent aggregation. This project aims to determine whether particular reactive oxygen species initiate α-synuclein misfolding and aggregation (Figure 1), and should reveal a better insight into the correlation between oxidative stress and sporadic α-synucleinopathies.

