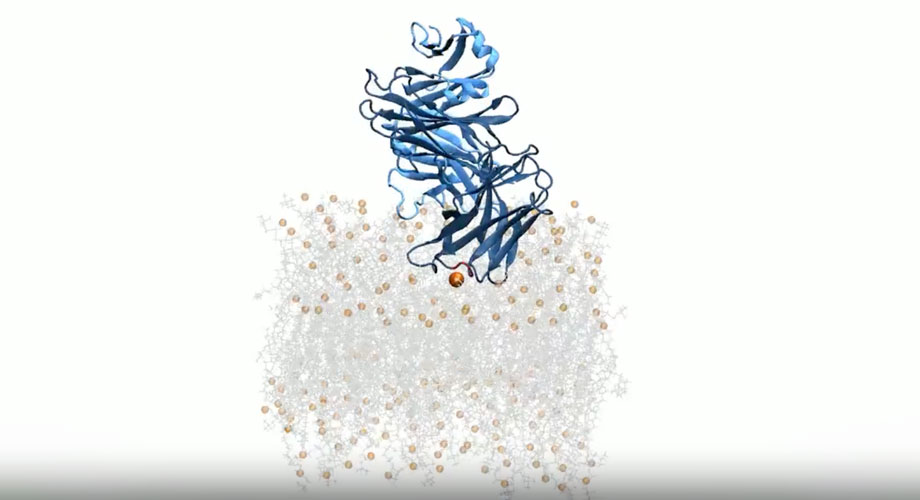
A simulation of a broadly neutralizing antibody (dark and light blue chains) binding to the HIV-1 viral membrane. The membrane comprises lipids and cholesterol (gray sticks) and phosphates (orange spheres). Credit: Scripps Research
Antibodies that recognize lipids: A new path for HIV vaccines and autoimmune disease treatments
Scripps Research scientists used computer modeling to illustrate how antibodies use fatty molecules known as lipids to recognize the HIV virus.
May 01, 2025
LA JOLLA, CA—Lipids are the fatty molecules that make up cellular membranes, creating a protective barrier that regulates what enters and exits the cell. Until recently, scientists believed antibodies couldn’t safely target lipids without risking harm to healthy tissues, since the same lipids that appear in viruses are widely distributed throughout the body.
Now, scientists at Scripps Research have used computer modeling to illustrate how a specific class of antibodies actually use lipids to recognize the HIV virus. Their work highlights antibody features that could help people design better vaccines for HIV and even autoimmune diseases.
“This work gives us a clearer picture of how these antibodies interact with the viral membrane, giving a blueprint we can potentially apply to vaccine design,” says Marco Mravic, an assistant professor at Scripps Research.
This study, published in eLife on April 7, 2025, focused on broadly neutralizing antibodies (bNAbs): a class of highly potent immune cells that latch onto a part of the HIV virus known as the membrane-proximal exposed region, or MPER. Researchers are interested in bNAbs for their potential to prevent and treat HIV, but it’s been difficult to understand how they recognize MPER because of where it’s located.
“This region sits right at the lipid bilayer—or membrane—on the HIV cells,” says first author Colleen Maillie, a joint PhD candidate in the labs of Mravic and Andrew Ward, who was a coauthor on this paper. “It’s notoriously difficult to reach because it’s concealed.”
The findings build on previous work conducted at Scripps Research: The labs of professors Michael Zwick and Dennis Burton identified the very rare antibodies targeting the important MPER epitope, and the labs of professors Ian Wilson and Ward used structural biology to illustrate that lipids could be important for bNAbs recognizing HIV and targeting the MPER.
In their new study, Mravic and his team wanted to dig deeper into these past discoveries. They used computer modeling to simulate how the antibodies approached and bound to the MPER. The models identified two key structural features on the antibodies. One was loop regions, highly variable regions on the antibody that are most responsible for their function and that recognize and bind to key sites on the virus. The other was framework regions, which form sheetlike structures on the surface of the virus’s membrane. These framework sections are hardwired from birth and were generally not thought to be important for binding. Such a finding supports the “germline targeting” strategy for HIV vaccine design pioneered at Scripps Research by Professor William Schief.
Using computational tools also gave the team an advantage over in-person experimentation because it’s difficult for researchers to work with proteins and lipids at the same time. Previous studies ignored the membranes to focus on MPER, Maillie explains, so researchers didn’t know how the antibody oriented relative to the membrane.
Researchers in this area have debated whether the antibody recognizes the lipid membrane component first and then diffuses across the viral surface to reach the MPER, or vice versa. The Scripps Research team’s work suggests the former is plausible, after simulating the antibodies in a bilayer made of just lipids. And this evidence helps them better understand how these antibodies target lipids.
Maillie was also surprised by how accurately the team reproduced the atomic-level interactions in their simulations.
“It was really cool to see that these physics-based approaches in our simulations are able to accurately model what we see in nature through experimentation,” she says. “If we have that accuracy, what else can we start to determine about these antibodies?”
One such question is how antibodies develop these lipid-targeting traits in the first place. The body needs to avoid targeting the lipids in its own membranes, so traditionally, any increase in lipid affinity has been linked with autoimmune risk.
But when the team modeled antibodies at different stages of maturity, they saw that the antibody’s affinity for the lipid component on the HIV virus increased as the antibodies matured.
“That was somewhat shocking—that the immune system is tolerating these increased affinities for the membrane,” she says.
Specificity may be the key. Autoimmune diseases are associated with less-specific interactions between antibodies and lipids. On the other hand, these newly studied antibodies seem to target well-defined membrane features—an interaction the team mapped to atomic-level detail.
“The structure of the antibody itself holds an encoding principle to recognize lipids, which is something that was underappreciated before,” Maillie says.
Understanding these structural determinants could open new doors in both vaccine research and synthetic antibody design. For HIV, researchers are designing antigens that trigger the production of antibodies that can target the MPER region with both protein and lipid specificity, just like bNAbs do.
Beyond HIV, the findings could have implications for diseases such as lupus, where antibodies target the body’s own membranes.
“It’s really interesting to see how this lipid-recognition principle might differ within other detrimental diseases, where antibody maturation plays a key role,” Maillie says.
The researchers also believe these insights could guide efforts in antibody engineering, potentially yielding synthetic antibodies that can recognize complex proteins in the cellular membrane like ion channels or G-coupled protein receptors, which are desirable targets in drug development.
“Synthetic protein engineers can look at this and say, ‘The immune system tolerates these specific lipid-targeting elements,’” Maillie says. “Maybe other antibodies can start to incorporate those and use them to their advantage.”
“Ab initio prediction of specific phospholipid complexes and membrane association of HIV-1 MPER antibodies by multi-scale simulations” was coauthored by Colleen A. Maillie, Kiana Golden, Ian A. Wilson, Andrew B. Ward, and Marco Mravic.
Support for the research came from the National Institute of Allergy and Infectious Diseases (UM1 AI144462).
For more information, contact press@scripps.edu

