Learning Page
This Learning Page describes interesting concepts and methods relevant to our work – and more generally, the field of biophysics as it relates to biology and disease. The articles are written by students and post-docs from the lab and provide a flavor for these topics. We hope that they will inspire you to learn more about our work.
The page is a work in progress and will be updated regularly. Please feel free to share it with others.
- Ashok
Blacklights and Biophysics: How Fluorescence Works
Emily Bentley
Ever seen something glow under a blacklight? That’s fluorescence – the way certain materials absorb and release light.
Fluorescence happens fast, in nanoseconds. (Phosphorescence is a slower phenomenon that can store light for hours. That’s how most glow-in-the-dark things work.) Both processes arise from the clouds of electrons that orbit atoms. When an electron absorbs light, it increases in energy and becomes excited. It can lose that energy through vibration, creating heat – or it can relax back to its low-energy state by releasing light.
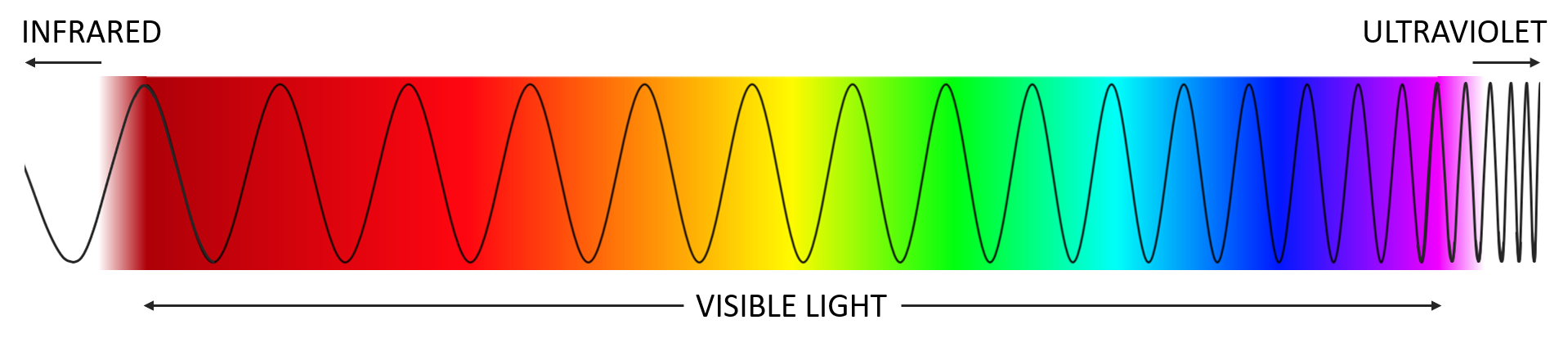
Light comes in a range of energies that determine its color. Red light transmits low energy in wide waves. At the other end of the rainbow, violet light carries high energy in short, quick waves. Next comes ultraviolet light – the kind emitted by a blacklight. Ultraviolet light has more energy than any light visible to us.
When a fluorescent material absorbs light, it gains the light’s energy, but it loses some of that energy as heat. When its electrons release the remaining energy, the resulting light has lower energy than the light originally absorbed. In other words, the color of light will move toward the red end of the rainbow. For example, one common fluorescent dye absorbs blue light and releases green light.
Back to blacklights. Our eyes never evolved to detect ultraviolet light, so it is invisible to us. Not to electrons, though! After an electron absorbs ultraviolet light, it relaxes and releases light with lower energy – visible light. That fluorescence causes the eerie blacklight glow that, to the human eye, appears from nowhere.
Our lab uses fluorescence tools to study how biological molecules move, interact, and change shape. Check out our research and publications to learn more, or check back on this page to see more articles like this one in the future.
Updated 9/19/2018