Research Program
1. Introduction
Viral chronic infections in the central nervous system (CNS) can cause progressive neurologic disorders associated with diverse pathology. These findings have led to the hypothesis that viruses can contribute to a variety of human mental diseases whose etiologies remain elusive. Therefore, the elucidation of the mechanisms by which viruses persist in the CNS and affect brain function is of paramount importance in biomedical research. My research program is focused on understanding the molecular and cellular mechanisms underlying non-lytic viral persistent infections of the CNS and associated disorders, as well as exploring new therapeutic approaches to combat such infections.
To investigate these questions, we use two viral model systems, lymphocytic choriomeningitis virus (LCMV) and Borna disease virus (BDV). Both BDV and LCMV persist in the CNS and cause cognitive and behavioral abnormalities associated with specific neurobehavioral, neurochemical and neurodevelopmental disturbances. Studies on these viral systems are contributing to the elucidation of: (i) the effect of environmental influences on the developing CNS and (ii) virally induced disturbances in cell differentiated functions that maintain brain homeostasis.
A comprehensive understanding of viral persistence in the CNS requires a detailed knowledge about the mechanisms involved in the control of virus replication and gene expression. Consequently, a main component of my research program is aimed at the investigation of the molecular and cellular biology of BDV and LCMV.
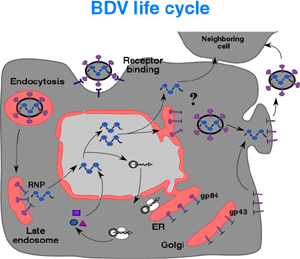
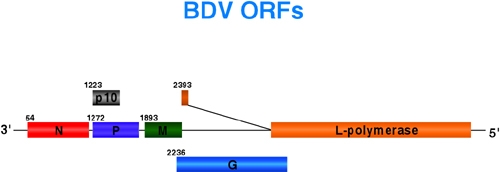
2. Molecular and cell biology of BDV and LCMV
2.1. Overview
BDV and LCMV have nonsegmented and bisegmented, respectively, negative-strand RNA genomes. Direct manipulation of RNA virus genomes depends on the ability to produce recombinant RNAs that are accepted as template by the particular viral RNA-dependent RNA polymerase (RdRp).
Polymerases of negative strand (NS) RNA viruses use as a template a nucleocapsid (NC) consisting of the genomic RNA tightly encapsidated by the virus nucleoprotein (N). The NC associated with the virus polymerase form a ribonucleoprotein (RNP) complex. This RNP complex is the minimum unit of virus infectivity. In contrast to positive-stranded RNA viruses, deproteinized genomic and antigenomic RNAs of NS RNA viruses cannot function as mRNAs and are not infectious. Thus, generation of biologically active synthetic virus from cDNA requires trans complementation by all viral proteins involved in virus replication and transcription. These considerations have severely hindered the application of recombinant DNA technology to the genetic analysis of these viruses. However, significant progress has recently been made and for several NS RNA viruses, short model genomes could be encapsidated and expressed by plasmid-encoded proteins. This approach allows for the analysis of cis-acting sequences and trans-acting factors required for virus replication, transcription, assembly and budding. Moreover, the use of reverse genetic approaches has allowed the rescue of infectious NS RNA viruses entirely from cloned cDNAs. This achievement has opened the possibility for direct genetic manipulation of the genomes of NS RNA virus, which represents a revolutionary breakthrough in virology.
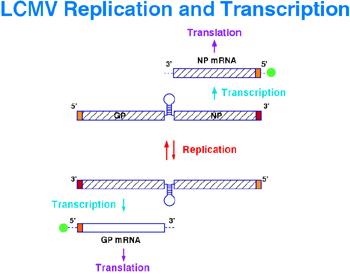
2.2. Arenavirus reverse genetics
LCMV is the prototypic member of the family Arenaviridae, viruses with a bisegmented negative strand RNA genome that include clinically important human pathogens such as Lassa fever virus and several newly recognized viral hemorrhagic fevers. We have developed a reverse genetic system for LCMVthat provides us with a novel and powerful approach for the investigation of cis-acting signals and trans-acting factors involved in arenavirus RNA replication, transcription, assembly and budding.
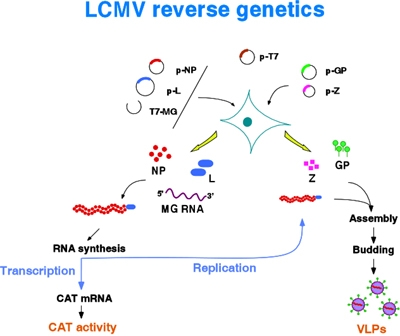
Our system is based on the use of synthetic virus minigenomes, or minireplicons, that allow for the amplification and expression of reporter genes mediated by expression of viral polypeptides provided from cloned cDNAs. The LCMV minigenome system that we have established is very robust, and besides reporter gene activity, either CAT or GFP, we can also directly detect the predicted subgenomic mRNA and progeny antigenome RNA species resulting from transcription and replication, respectively. We are now in a position to conduct a detailed molecular characterization of the cis-acting signals controlling LCMV RNA synthesis and to probe the function of viral polypeptides in virus replication, control of gene expression, assembly and budding, as well as their interactions with host cell factors. Thus, using this system we have determined the minimal cis-acting signals and viral trans-acting factors required for arenavirus RNA replicaion and transcription. The virus RdRp, L protein, and nucleoprotein (NP) are the minimal trans-acting viral factors required for arenavirus RNA synthesis mediated by the LCMV polymerase. We have also demonstrated that the assembly and budding of LCMV infectious particles requires, in addition to L and NP, the virus glycoprotein (GP) and the small RING finger protein Z.
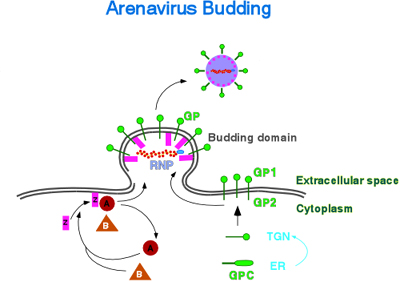
Arenaviruses include members that are highly pathogenic for human, such as Lassa fever virus. Our LCMV minigenome system provides us with a platform to develop robust assays to assess new antiviral strategies against this group of viruses. Using the LCMV minigenome system we have found that Z has a very powerful inhibitory effect on RNA synthesis mediated by the LCMV polymerase. Transduction of cells with a replication deficient recombinant adenovirus expressing LCMV Z confers complete protection against LCMV infection The elucidation of the molecular mechanisms underlying this property of Z may lead to the design of new antiviral strategies against arenaviruses. We have also identified Z as the driving force for LCMV budding. This process appears to be mediated by the overlapping PT/SAP and PPXY motifs located at the C-terminus of arenavirus Z proteins. These motifs have also been shown to control budding of HIVand Ebola virus via interaction with cellular proteins of the vesicular protein-sorting (Vsp) machinery and the multivesicular bodies (MVB) pathway. The elucidation of the molecular details of Z interactions with cellular components required for cell exiting, such as the MVB proteins Tsg101 and Nedd4, should facilitate the design of small-molecules inhibitors with potential antiviral activity against highly pathogenic arenaviruses, as well as other lethal human viruses using similar pathways for exiting the infected cell. We will use cellular, biochemical and genetic assays to identify Z-interacting cellular proteins, as well as to characterize the domains involved in these interactions. Cellular proteins identified as Z-interacting partners required for budding will be used to develop proof-of-concept biochemical and fluorescence resonance energy transfer assays for the screening of small molecules that might interfere with virus budding. These assays will be adapted to a semi-high throughput format and be used for the screening of combinatorial chemical libraries to identify small molecules capable of interfering with these protein-protein interactions. Candidate molecules will be tested in cultured cells using inhibition of LCMV growth as an endpoint. Molecules with inhibitory activity in cultured cells and exhibiting acceptable levels of toxicity will be tested in a mouse model of LCMV infection.
2.3. Molecular biology of BDV
BDV is a nonsegmented negative-stranded (NNS) RNA virus with a genome organization characteristic of Mononegavirales. However, based on its unique biological and genetic features, BDV is considered to be the prototypic member of a new virus family. Therefore, studies with BDV will provide new insights about the biology of NS viruses that might not be obtained from studies with other prototype NS RNA viruses for which reverse genetic systems are already established. For the investigation of the molecular biology of BDV we are also developing a reverse genetic system. The efficiency of the BDV reverse genetic system is still low and we are working on its optimization to exploit its great potential to investigate the molecular biology of BDV. Our efforts are aimed to the understanding of: (i) the mechanisms involved in the control of BDV gene expression and RNA replication, and (ii) the regulation of the nucleocytoplasmic transport of BDV macromolecules and RNP complexes.
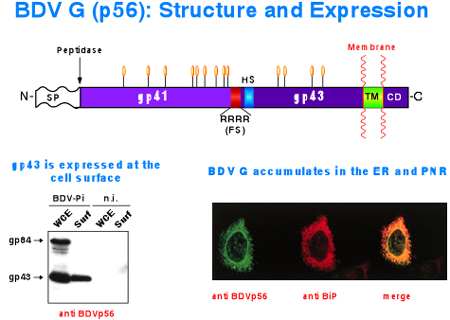
BDV exhibits an exquisite affinity for neurons of the limbic system, especially for those in the hippocampal formation. As with other NS RNA virus, this tropism is expected to be mediated by the virus surface glycoprotein (GP). Hence our great interest in determining the role of the BDV GP in virus tropism, as well as in the identification and characterization of the cellular receptors used by BDV. These investigations are hampered by the lack of cell-free virus associated with BDV infection. To overcome this obstacle, we have generated a recombinant VSV where the VSV GP was replaced by the BDV GP. To facilitate monitoring infectivity, the ORF of the GFP was also included into the recombinant virus. This recombinant virus (VSVr/BV-GP) exhibits the characteristic tropism of BDV both in primary hippocampal cell cultures and rodent brain. We will use this newly developed tool to search for BDV cellular receptors.
Both BDV and LCMV infections are manifested as complex spectrums of host symptoms. This finding, illustrates the delicate balance in which virus-host interaction hang, and how minor differences in either the host or viral genetics can profoundly influence the outcome of infection. Important findings in this research area have been made based on the very laborious, and limited, approach of identification, isolation and characterization of naturally, and rarely, occurring LCMV and BDV mutants exhibiting phenotypes of interest. The ability to generate predetermined specific mutations within the BDV and LCMV genomes, and analyze their phenotypic expression, will provide a new powerful approach for the elucidation of the molecular mechanisms underlying virus-host interactions, including the bases of viral persistence and associated disease.
3. Molecular neurovirology
Another central component of my research program focuses on using LCMV and BDV to elucidate mechanisms whereby viruses interfere with specialized functions of brain cells, thus leading to disturbances in brain homeostasis and CNS disorders in the absence of the hallmarks of cytolysis and inflammation. Moreover, serological and molecular epidemiological studies indicate that BDV can infect humans, and is possibly associated with certain neuropsychiatric disorders.
Using a combination of approaches, including cultured cells and animal models, we have identified a variety of neuronal and astrocyte functions that are affected in LCMV and BDV persistently infected animals. We are pursuing biochemical and genetic approaches to dissect the molecular bases of these virus-cell interactions. Our aims are:
- Identification of the cellular and molecular pathways contributing to BDV and LCMV induced behavioral abnormalities.
- Elucidation of the cellular and molecular mechanisms underlying degeneration of the dentate gyrus, and altered cerebellar development associated with BDV infection.
- Examination of the role played by astrocytes in virally induced neuronal disturbances.
Astrocytes play a central role in maintaining brain homeostasis. BDVpersistence in the CNS causes a chronic and prominent astrocytosis. We have documented that primary cortical astrocytes persistently infected by BDV exhibit a severe and specific impairment in their ability to take up glutamate, thus altering an important physiological function for maintaining brain homeostasis.
In collaboration with G. Sutcliffe and his colleagues at DGT, we have used TOGA (Total Gene Expression Analysis) to compare the profiles of global CNS gene expression between BDV-infected and mock-infected rats. TOGA is an automated parsing technology for analyzing expression of nearly all mRNA species regardless of whether the gene is known.
TOGA analysis identified 506 genes whose expression is altered, up or down-regulated in the range of two-fold to as great as 100-fold in the CNS of BDV-infected rats. We have identified genes involved in a variety of cellular functions and metabolic pathways that can contribute to BDV-induced CNS disturbances. From the 506 targets identified, we have used a subset of 100 genes for validation purposes by using real time RT-PCR and Northern blot studies. We have found a validation rate of 98% (98 out of 100 genes examined). Moreover, we have identified several potential novel genes whose expression is altered in BDV-infected rats. We are currently doing similar studies on mice persistently infected with LCMV. Results from this �discovery driven� research has given us a holistic view of virally induced molecular changes in the CNS gene expression program of the infected host. This information, in turn, has provided us with the foundations for further �hypothesis driven� research aimed at the elucidation of molecular mechanisms by which viruses can affect CNS function in the absence of the classic hallmarks of cytolysis and immune cell infiltrates. Moreover, the identification and characterization of novel genes whose expression is altered by virus infection of the CNS, may provide us with new knowledge about virus-cell interactions in the CNS, and contribute to our understanding not only of viral persistence but also brain function.
4. A new approach to combat infections by RNA viruses
Riboviruses do not integrate into the host genome and have error prone replication systems. Hence, RNA viruses replicate and evolve as complex mutant distributions termed viral quasispeies. These features have lead to the view that persistent infections by RNA viruses can be viewed as a succession of invasions modulated by the response of the host. The health impact of these chronic infections will differ not only because of the genetic, physiological and immunological differences among hosts, but also because each host experiences a unique array of quasi-species challenges during infection.
Experimental data together with theoretical considerations indicate that replication fidelity of RNA viruses is close to the critical error threshold tolerated to maintain meaningful biological information. This proximity maximizes virus adaptability, but it also predicts that small increases in polymerase error frequency will force the viral system into error catastrophe with the �melting� of meaningful information through randomization of nucleotide sequences, resulting in loss of viral infectivity. We are using the LCMV system to explore the feasibility of this new approach against infections by riboviruses.
We have found that treatment of LCMV-infected cells with the mutagenic agent fluorouracil (FU) is highly effective in reducing the virus load, leading systematically to complete virus extinction. Treatment with FU resulted in increases of 3.5-to 10-fold in mutation frequency (3.5 for NP; 5.9 for L and 10 for GP). However, FU had only a very modest direct effect on levels of LCMV transcription and RNA replication, as well as on the expression of a LCMV minireplicon. These findings strongly support the conclusion that �lethal mutagenesis� is responsible for virus extinction. Consistent with these findings, we have observed that the establishment of LCMV persistent infection in mice deficient in B and T cells (RAG-/- mice) can be successfully prevented by treatment with FU.
Riboviruses exhibit a pertinacious adaptability reflected by the high-frequency isolation of escape mutants whenever an effective selective constraint is in operation. Our findings, together with those recently described for picornaviruses and HIV by other investigators, should encourage exploring whether entry into error catastrophe could be developed into a new antiviral strategy. Such strategy will be aimed at the design of viral-specific, fidelity lowering drugs that could be applied in combination with immunotherapy and inhibitors of viral replication. The investigation of this new approach for the treatment of infections by RNA viruses will be pursued using LCMV and BDV as model systems.

