The enhanced aromatic sequon and the energetics of N-glycosylation
Nearly a third of the eukaryotic proteome, including integral membrane proteins, extracellular proteins, and most proteins harbored in organelles, traverse the cellular secretory pathway. Most of these proteins are co-translationally N-glycosylated in the endoplasmic reticulum (ER) by oligosaccharyl-transferase (OST), which catalyzes the en bloc transfer of an oligomannose glycan onto the side chain amide NH of Asn residues within Asn-Xxx-Ser/Thr sequences, or sequons, where Xxx can be any amino acid other than Pro. N-glycans enable N-glycoproteins in the ER to get folding assistance from the dedicated glycoproteostasis network, including the calnexin and calreticulin chaperone pathways, which greatly enhance N-glycoprotein folding efficiency and trafficking through a so-called extrinsic mechanism. N-glycosylation can also intrinsically accelerate protein folding, slow protein unfolding, and increase protein thermodynamic stability through glycan–protein contacts.
The proteins used to investigate the intrinsic effects of N-glycosylation include the adhesion domain of human CD2 (HsCD2ad; N-glycosylation is required for it to fold) or rat CD2 (RnCD2ad; which is not normally N-glycosylated), human muscle acylphosphatase (AcyP2; which is cytoplasmic and therefore not normally N-glycosylated), and the WW domain of human Pin 1 (Pin WW; also cytoplasmic and not normally N-glycosylated). We wanted to define a set of criteria that could be used in the rational design of N-glycosylation sites that conferred stabilization to the glycoprotein upon N-glycosylation, rather than employ the current empirical approach of trial-and-error to identify sites of N-glycosylation that result in glycoprotein stabilization. These studies were done in collaboration with the Wong and Dyson laboratories. We have shown that:
- N-glycosylated HsCD2ad is –3.1 kcal/mol more stable and folds 4 times faster than nonglycosylated HsCD2ad. The first GlcNAc of the N-glycan accounts for 2/3 of this stabilization energy and the entire acceleration of folding, while the next two saccharides (GlcNAc2-Man) account for the remaining 1/3 of the stabilization free energy.
- Stabilization is mediated by a tripartite interaction between the side chains of Phe63, GlcNAc1 attached to Asn65, and Thr67 in HsCD2ad, a structural motif we named an enhanced aromatic sequon—a glycosylation sequon harboring an aromatic side chain two or three residues before the N-glycosylated Asn.
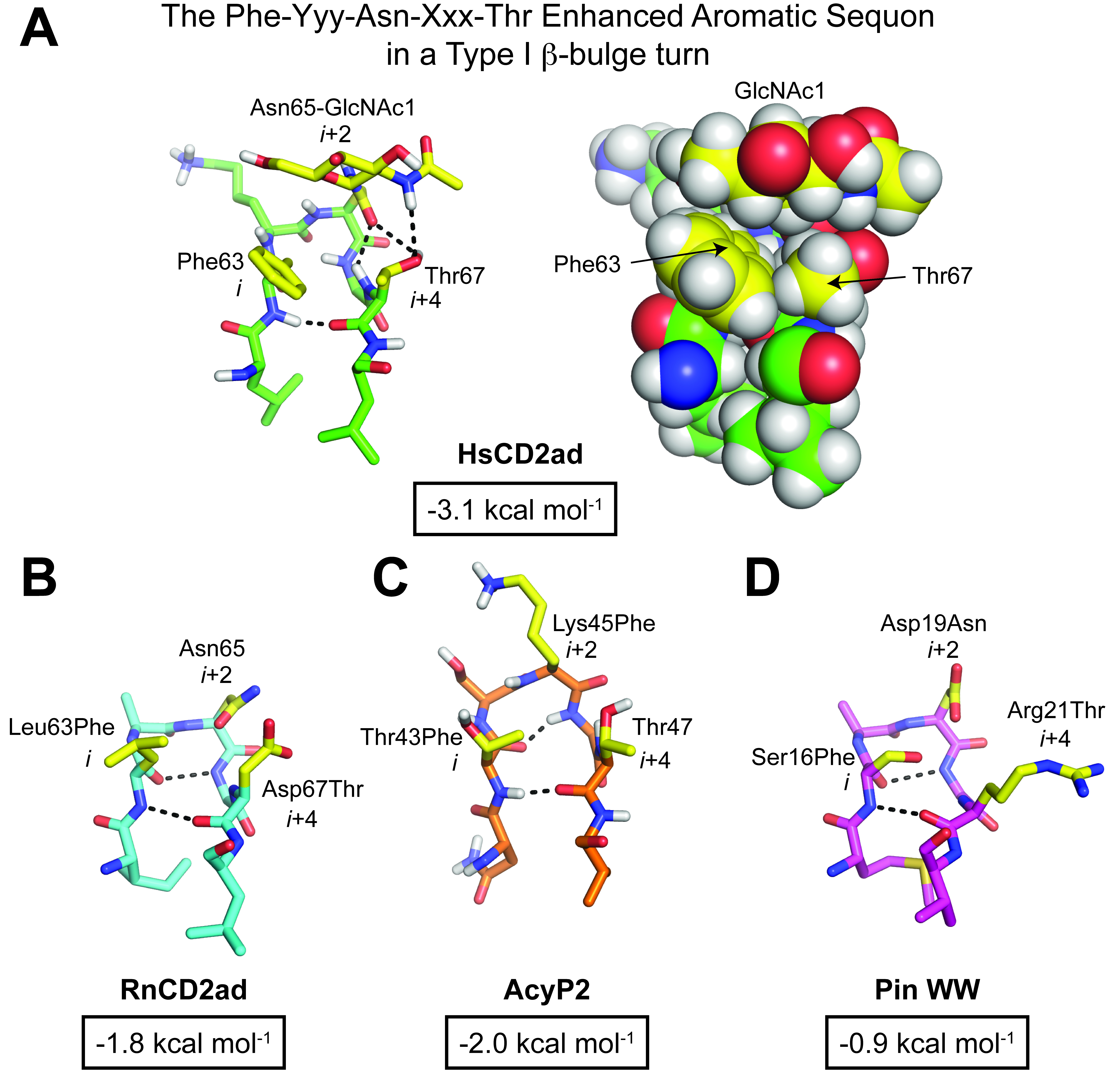
- Incorporation of this enhanced aromatic sequon into specific reverse turns in RnCD2ad, AcyP2 and Pin WW increased native state stability by between -0.7 to -2.0 kcal/mol upon N-glycosylation. The best reverse turn contexts for the enhanced aromatic sequon are: a five-residue type I β-turn harboring a G1 β-bulge (using a Phe-Yyy-Asn-Xxx-Thr sequon) and a type II β-turn within a six-residue loop (using a Phe-Yyy-Zzz-Asn-Xxx-Thr sequon).
- The NMR-based structures of these 5- and 6-residue enhanced aromatic sequons demonstrate that the α-face of GlcNAc1 packs against the aromatic ring at the n-2 or n-3 position relative to the glycosylated Asn, stabilizing the protein.
- N-PEGylation of a reverse turn is stabilizing in most sequence contexts owing to denatured state destabilization, unlike N-glycosylation which stabilizes the native state by a specific GlcNAc-aromatic face-to-face interaction.
- The α-face of GlcNAc1 interacting with the aromatic side chain at the n-2 or n-3 position is stabilizing due to the hydrophobic effect supplemented by CH–π
- Enhanced aromatic sequons increase OST glycosylation efficiency and glycan homogeneity. Any aromatic residue at n-2 in an enhanced aromatic sequon increases glycan occupancy in human cells, and this effect is dependent upon OST substrate preferences rather than differences in other cellular processing events, such as synthesis, degradation or trafficking alterations. Aromatic residues at n-2 relative to Asn alter glycan remodeling in the Golgi by making the sites less accessible for modification, producing proteins with less complex N-glycan structures.
- Variation of the stereochemistry and composition of GlcNAc1 showed that carbohydrate–aromatic interaction strengths are moderately affected by changes in the stereochemistry and identity of the substituents on the pyranose rings of the sugars. Galactose seems to make the weakest and allose the strongest sugar–aromatic interactions. GlcNAc, the sugar favored by evolution, falls in between galactose and allose. Peracetylation of the monosaccharides substantially increases the strength of the sugar–aromatic interaction in the context of the five–residue β-bulge enhanced aromatic sequon in Pin WW.
- Installing the enhanced aromatic sequon into the conserved N-glycosylation site in human IgG1 in a type I β-turn with a G1 β-bulge stabilized the human IgG1 Fc fragment. Stabilization is the result of both removing the side chain of a solvent exposed Tyr296 residue and introducing Phe at n-2 to replace the wild-type Gln295. In addition to the expected GlcNAc1-Phe293 interaction, there is also a stabilizing interaction between Phe295 and the fucose attached to the GlcNAc via an α1,6 linkage. Altering the environment of the N-glycan also changed the affinity of the Fc fragment for Fc receptors, which mediate the effector functions of antibodies
Currently, we are evaluating whether the major influence of dispersion and the minor contributions of electrostatics to the CH-π interaction is a consequence of using Phe, and not a more polarizable aromatic side chain, like that comprising Trp or Tyr. It also appears that the n-4 position contributes to N-glycosylation-associated stabilization in the context of human CD2ad, and this will be explored further in insect cells in combination with CD2ad mutagenesis. Additional ongoing studies include utilizing enhanced aromatic sequons along with complementary monosaccharide N-glycosylation to nucleate β-sheet formation as an approach to create hyperstable small proteins for pharmacologic and research applications.

