
Protocols
Preparation of Human PMN Cells
(Protocol from StemCell Technologies)
Materials: HetaSep (#07806: 20 ml or #07906: 100 ml), EasySep Human Neutrophil Enrichment Kit (#19257) and Falcon 5 mL Polystyrene tube to properly fit into the Purple EasySep magnet (#352058)
Wash medium: DPBS free of Mg2+ and Ca2+ + 2% FBS + 1 mM EDTA
Preparation of HetaSep-Treated Blood:
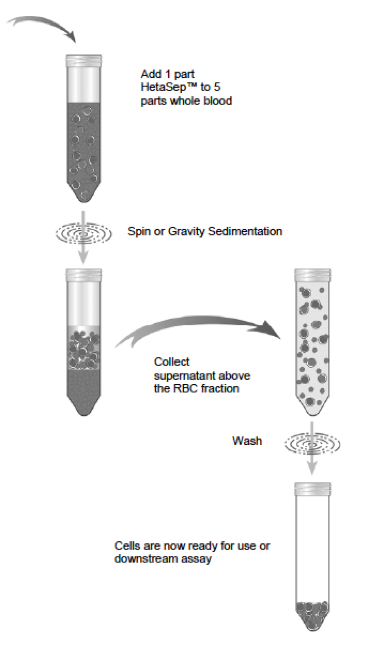
- Add 1 part HetaSep to 5 parts whole blood (2 ml HetaSep to 10 ml blood). Mix well.
- Centrifuge sample at room temperature at 110 x g with the brake off for 6 min.
- Remove sample from centrifuge and allow to sit undisturbed at room temperature for 10 min (until the plasma layer represents 40-50% of the total volume). This will allow further sedimentation of the RBC and will improve recovery of the nucleated cells.
- Carefully harvest the leukocytes-rich supernatant into a fresh 50 ml tube (everything above the red blood cell pellet). Up to 5-10% of the initial red blood cells may not have sedimented and thus may still remain in this fraction. This is expected.
- Wash this fraction with at least a four-fold volume of Wash medium. This may require several tubes. Centrifuge for 10 min at room temperature at 500 x g with the brake set to low.
- Discard supernatant and wash a second time to remove excess platelets by centrifuging for 10 min at 120 x g with the brake off.
- Remove supernatant carefully and resuspend the cells in a small volume of Wash medium. Count the cells.
EasySep Protocol Using the EasySep Magnet:
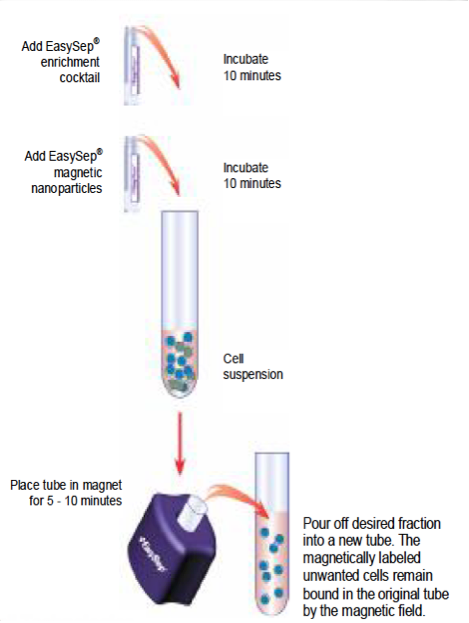
- Prepare nucleated cells suspension at a concentration of 5 x 107 cells/ml in Wash medium.
- Place 2 mL of cells in a 5 ml polystyrene tube.
- Add 100 μl of EasySep Human Neutrophil Enrichment Cocktail to the tube containing cells. Mix well and incubate at room temperature for 10 min.
- Mix EasySep Nanoparticles to ensure that they are in a uniform suspension by vigorously pipetting more than 5 times. Vortexing is not recommended. Add 200 μl of nanoparticles to the cells. Mix well and incubate at room temperature for 10 min.
- Bring the cell suspension to a total volume of 2.5 ml by adding 200 μl of Wash medium . Thoroughly mix the cells to disrupt any red blood cell aggregates by gently pipetting up and down. Place the tube (without cap) into the magnet. Set aside for 5 min.
- Pick up the EasySep Magnet, and in one continuous motion invert the magnet and tube, pouring off the desired fraction into a new 5 ml polystyrene tube. The magnetically labeled unwanted cells will remain bound inside the original tube, held by the magnetic field of the magnet. Leave the magnet and the tube in inverted position for 2-3 sec, then return to upright position. Do not shake or blot off any drops that may remain hanging from the mouth of the tube.
- Remove the empty tube from the EasySep magnet and place the new tube containing the supernatant fraction into the magnet. Set aside for 5 min.
- Repeat Step 5. The negatively selected enriched cells in the new tube are now ready for use.

