Pushing the Limits of Lower-Cost Electron Microscopes, with Incredible Results
By Bonnie Ward and Madeline McCurry-Schmidt
From Ebola virus’s deadly machinery to crucial human cell receptors, recent technical breakthroughs in cryo-electron microscopy (cryo-EM) have allowed scientists to obtain unprecedented insights into a variety of molecular structures directly involved in health and disease pathologies.
In fact, this technology—which gives researchers detailed three-dimensional views of biomolecules in near-native states—recently drew the eye of the Nobel Prize committee, which awarded the 2017 Prize in Chemistry to three founding members of the field of cryo-EM. The field has seen an explosive development over the past few years, both in terms of technical and methodological advances. Nowadays, scientists can reliably use this technique to visualize critical disease-relevant biomolecules, and the 3D structures of solved by cryo-EM are a cornerstone of scientific literature.
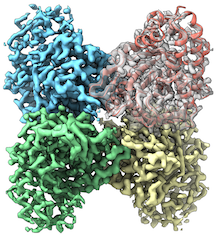
At The Scripps Research Institute (TSRI), Associate Professor Gabriel Lander, Ph.D., leads a team pushing the limits of cryo-EM technology to further improve and expand the utility of this technique. His lab’s most recent work, published in Nature Methods, demonstrates that TSRI researchers—and the scientific community as a whole—can visualize astonishing molecular details of biomolecules that were previously thought to be too small to be resolved. What’s more, they can accomplish this even on less expensive electron microscopes.
“This work has reshaped the way the EM field views these microscopes,” said Lander. “Hopefully research institutes and universities will realize that, without a massive investment, they can do a lot of this work themselves,” he said.
In their study, Lander and colleagues showed that resolutions of similar quality could be achieved using the 200-keV transmission electron microscope (TEM) versus the significantly more expensive 300-keV TEM.
“TSRI was one of the first institutes in the world to purchase this ‘mid-range’ electron microscope, and we have shown that it can be used to solve structures at resolutions that were previously only thought attainable using top-of-the-line ‘Titan Krios’-type microscopes,” said Lander.
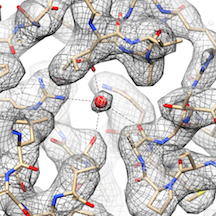
The resolution of a structure is important because higher resolutions provide scientists with clear images of molecular interactions in minute detail that can be confidently applied towards drug development. “Cryo-EM lets us examine the machinery of cells at the atomic level,” said Mark Herzik, Ph.D., the study’s first co-author and a Helen Hay Whitney Foundation postdoctoral fellow in Lander’s lab. “This level of detail can aide researchers in designing drugs based on blocking certain cellular activities for therapeutic purposes,” he said, noting this approach is known as structure-based drug design.
Lander and his team’s desire to move forward with such research as quickly as possible led to their new discovery.
TSRI purchased a high-powered 300-keV TEM three years ago and researcher interest quickly grew—and so did the wait list to use the equipment. “It took about three months to schedule a 24-hour time slot on the microscope,” said Lander.
This led TSRI to purchase a mid-level cryo-EM microscope (a 200-keV TEM) for the specific purpose of paring down cellular samples to the best candidates. TSRI bought the intermediate microscope last year, and Lander and his team soon got surprising results.
“We never expected to be able to resolve structures at this resolution on this microscope,” said Lander. “That really inspired us to try to push the resolution levels even higher. Through very careful sample preparation and microscope alignments, we ended up getting resolutions that were comparable to the higher-end model (300-keV TEM).”
Based on this discovery, TSRI researchers can now make greater use of cryo-EM. “We’ve essentially doubled the number of microscopes that can turn out high-resolution images 24-7,” said Lander. Together with improved data collection strategies, wait times also have vastly improved, going from three months to approximately one to two weeks for a microscope time slot, he said.
Along with improving access for TSRI researchers, Lander and his team hope their finding will boost cryo-EM research across the scientific community.
“Understanding disease, and how to develop therapeutics to effectively treat disease, is predicated on having very detailed structural information,” said study co-author Mengyu Wu, a graduate student in Lander’s lab. “Cryo-EM has become one of the most powerful methods for obtaining these critical insights.”
Send comments to: press[at]scripps.edu