Study Finds Emerging Bird Flu Strain Is Still Poorly Adapted for Infecting Humans
Avian influenza virus H7N9, which killed several dozen people in China earlier this year, has not yet acquired the changes needed to infect humans easily, according to a new study by scientists at The Scripps Research Institute (TSRI). In contrast to some initial studies that had suggested that H7N9 poses an imminent risk of a global pandemic, the new research found, based on analyses of virus samples from the Chinese outbreak, that H7N9 is still mainly adapted for infecting birds, not humans.
“Luckily, H7N9 viruses just don’t yet seem well adapted for binding to human receptors,” said Ian A. Wilson, the Hansen Professor of Structural Biology and chair of the Department of Integrative Structural and Computational Biology at TSRI.
“Because publications to date have implied that H7N9 has adapted to human receptors, we felt we should make a clear statement about this,” said James C. Paulson, chair of TSRI’s Department of Cell and Molecular Biology.
The Wilson and Paulson laboratories collaborated on the study, which is reported in the December 6, 2013 issue of the journal Science.
A Worrisome Outbreak
H7N9 flu viruses infect birds, apparently causing them few or no symptoms. Until this year these strains had never been reported in humans. However, starting in February in two urban areas of eastern China, dozens of people began to come down with H7N9 flu. Most became severely ill. By the end of May, when the outbreak had mostly subsided, there were 132 laboratory-confirmed human cases and 37 deaths—a nearly 30% case-fatality rate.
The outbreak understandably alarmed public health officials, and across the globe dozens of laboratories began studying H7N9 isolates from infected patients. The big question was whether these strains were capable of spreading only in a limited, sporadic way from birds to humans—many of the cases were linked to poultry exposure—or if they had truly “jumped the species barrier.” If the latter were true, and H7N9 could now spread from human to human, the Chinese outbreak might be the start of a global pandemic.
Some prominent early studies came to worrisome conclusions. For example, most of the H7N9 isolates from the outbreak turned out to have acquired a notorious flu-virus mutation that substitutes the amino acid leucine for glutamine in the part of the virus that grabs receptors on host cells. The same mutation, in other influenza virus subtypes, was apparently a key enabler of pandemics that killed an estimated one million people worldwide in 1968-69 (the “Hong Kong flu”) and two million during 1957-58 (the “Asian flu”). Initial studies of the new H7N9 isolates in mice, ferrets and monkeys also suggested that they had at least a limited ability to spread among mammals.
Answering Critical Questions
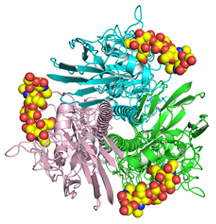
Paulson’s and Wilson’s laboratories, long experienced in flu virus and immunity research, were among the many that mobilized to try to answer the crucial question of H7N9’s transmissibility among humans. They quickly decided to collaborate. Paulson’s laboratory evaluated H7N9’s ability to bind the sialylated sugar receptors to which flu viruses normally attach on host cells. Wilson’s laboratory used X-ray crystallography to determine the atomic structures of the H7N9 hemagglutinin protein bound to these sialic acid receptor molecules.
Paulson’s team—including postdoctoral fellows Robert P. de Vries and Corwin M. Nycholat and Research Assistant Ryan McBride—tested the ability of the virus’s hemagglutinin (HA) protein, to bind to different human and avian receptor variants. These tests showed clearly that the isolate tested (A/Shanghai/2/2013 or “Sh2”) still has a strong preference for avian-type receptors and binds human-type receptor variants only weakly.
In Wilson’s laboratory, postdoctoral fellow Rui Xu, the study’s first author, along with Staff Scientist Xueyong Zhu and Research Assistant Wenli Yu, performed X-ray crystallography studies of the Sh2 HA protein bound to several avian- and human-type receptors. The latter, provided by Paulson’s laboratory, were more accurate versions of these receptors than any that had been used in previous H7N9 structural analyses.
The new data highlighted the looseness of the contacts that Sh2 HA makes with human-type receptors, in contrast to the snug couplings it makes with certain avian-type receptors.
Thus, despite hints that it had begun to adapt to human hosts rather than its natural bird hosts, H7N9 does not appear to pose an imminent threat of a human pandemic. “These results suggest that we should continue to observe H7N9 and see if it undergoes any changes that make it more likely to spread in the human population,” said Wilson. Paulson added, “If it does evolve a true human-type receptor specificity, it could potentially spread among humans much better than it does now.”
The study, “Preferential Recognition of Avian-Like Receptors in Human Influenza A H7N9 Viruses,” received support from the National Institutes for Health (R56 AI099275), the Skaggs Institute for Chemical Biology, the Scripps Microarray Core Facility, the Centers for Disease Control and the Netherlands Organization for Scientific Research. For additional information see http://www.sciencemag.org/content/342/6163/1230.abstract
Send comments to: press[at]scripps.edu