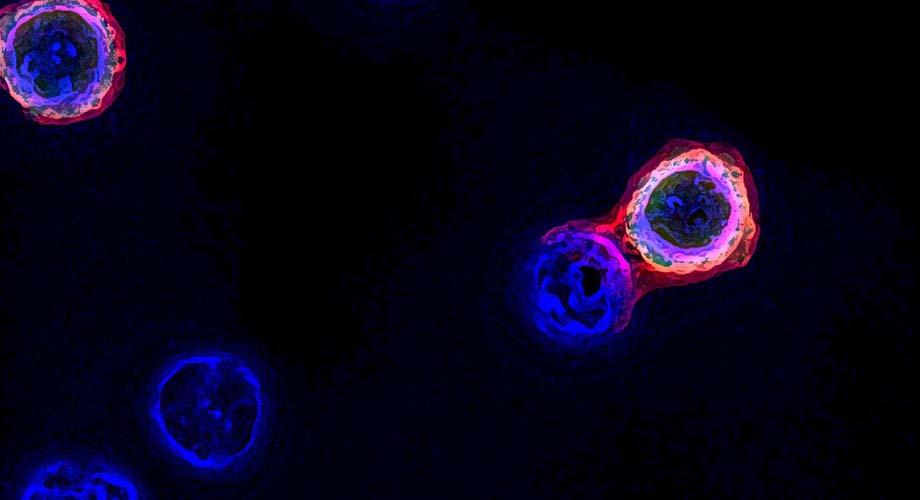
A cell infused with a special enzyme interacts with a tumor-targeting immune cell—a process that enables the immune cells to be easily captured and leveraged for a cancer treatment. (Image courtesy of the Wu laboratory at Scripps Research.)
Rapid method of isolating tumor-targeting T cells could propel personalized cancer treatments
A technique developed at Scripps Research seeks to fill an unmet need among scientists and doctors who need faster, more precise tools to identify useful tumor-infiltrating immune cells.
October 22, 2020
LA JOLLA, CA—When it comes to defeating cancer, some immune cells are mightier than others. But even the best-trained eye and today’s advanced scientific tools have trouble discerning the most powerful tumor-fighting cells from the rest.
A new technique developed by Scripps Research scientist Peng Wu, PhD, aims to change that—offering a new platform that could propel personalized cancer treatments that have been hindered due to the challenges of isolating the most useful immune cells in patients. The development is published October 22 in Cell.
“In many new and emerging personalized cancer therapies, the key to success is finding the sometimes-elusive T cells that are directly targeting the tumor, then creating more of those cells outside of patients’ bodies and re-introducing them for tumor treatment,” says Wu, associate professor in the Department of Molecular Medicine and senior author of the study. “With our simple method to detect and isolate tumor-reactive immune cells, my hope is that we can advance personalized immunotherapy treatments that are now either too costly or laborious to reach their potential.”
The method is called FucoID, named after the enzyme fucosyltransferase that plays a starring role in “tagging” the surface of sought-after immune cells so they can be seen and captured. The enzyme is loaded onto dendritic cells, a type of immune cell that presents tumor-specific material to the desired T cells. When the cells interact, the enzyme transfers a tag to the tumor-fighting cells so scientists can detect them with a fluorescent probe and extract them from the sample.
In experiments involving mice, the approach successfully identified multiple types of so-called “tumor antigen-specific T cells,” including CD4+ and CD8+ T cells that infiltrate tumors and attack from within. These cells are central to certain cancer immunotherapies—including checkpoint inhibitors and treatments known as adoptive TIL (tumor infiltrating lymphocyte) transfer therapies.
“This approach removes a significant barrier to studying tumor-specific T cells and will be immensely useful for both basic scientists and clinicians,” says John Teijaro, PhD, associate professor in the Department of Immunology and Microbiology and co-author of the study.
“This study also highlights how the highly collaborative environment at Scripps Research fosters innovative solutions to intractable problems.”
The FucoID process of isolating the appropriate cells takes only one day, compared with four or five weeks using current methods, according to Wu. “Once we isolate them, we can expand them into millions or billions of cells to construct a treatment or simply to study them,” he says.
Having the ability to quickly take stock of these cells in a patient can also help doctors predict therapeutic success or treatment progress, he says. And doing any of these things faster than today’s methods, which rely on bioinformatics or genetic manipulations, can make a big difference to patients.
Wu is now collaborating with clinicians at UC San Diego to use FucoID to isolate the desired T cells from human patient tumor samples, with the goal of eventually applying the platform to a clinical trial for a cancer treatment.
“We believe FucoID has potential to be translated to a clinical setting for the detection and isolation of tumor-reactive immune cells, ultimately paving the way for lowering the cost and accessibility of personalized cancer treatment,” Wu says.
The study, “Detecting Tumor Antigen-specific T cells via Interaction DependentFucosyl-biotinylation,” is authored by Zilei Liu, Jie Li, Mingkuan Chen, Mengyao Wu, Yujie Shi, Wei Li, John Teijaro and Peng Wu.
The research was supported by the National Institutes of Health (AI143884, GM093282) and the National Natural Science Foundation of China (81602091).
For more information, contact press@scripps.edu

