Welcome to the Gallay Laboratory
Hepatitis B Virus (HBV) Cure Overview
Click here for the Human Immunodeficiency Virus-1 (HIV-1) Cure Overview
Hepatitis B virus (HBV) is a main worldwide threat with over 257 million people chronically infected, and with over 887,000 deaths per year. Chronic hepatitis B causes 40% of cases of hepatocellular carcinoma, which is the second leading cause of cancer-related mortality worldwide. The current vaccine has no beneficial effect on established chronic infection. Current therapeutic treatments suppress HBV replication, but are not curative, due to the persistence of the viral covalently closed circular DNA (cccDNA) transcriptional template in infected hepatocytes in addition to the failure of chronically infected patients to develop an immune response robust and sustained enough to totally clear the infection. Therefore, most of the patients cannot interrupt their drug treatments for their entire lives.
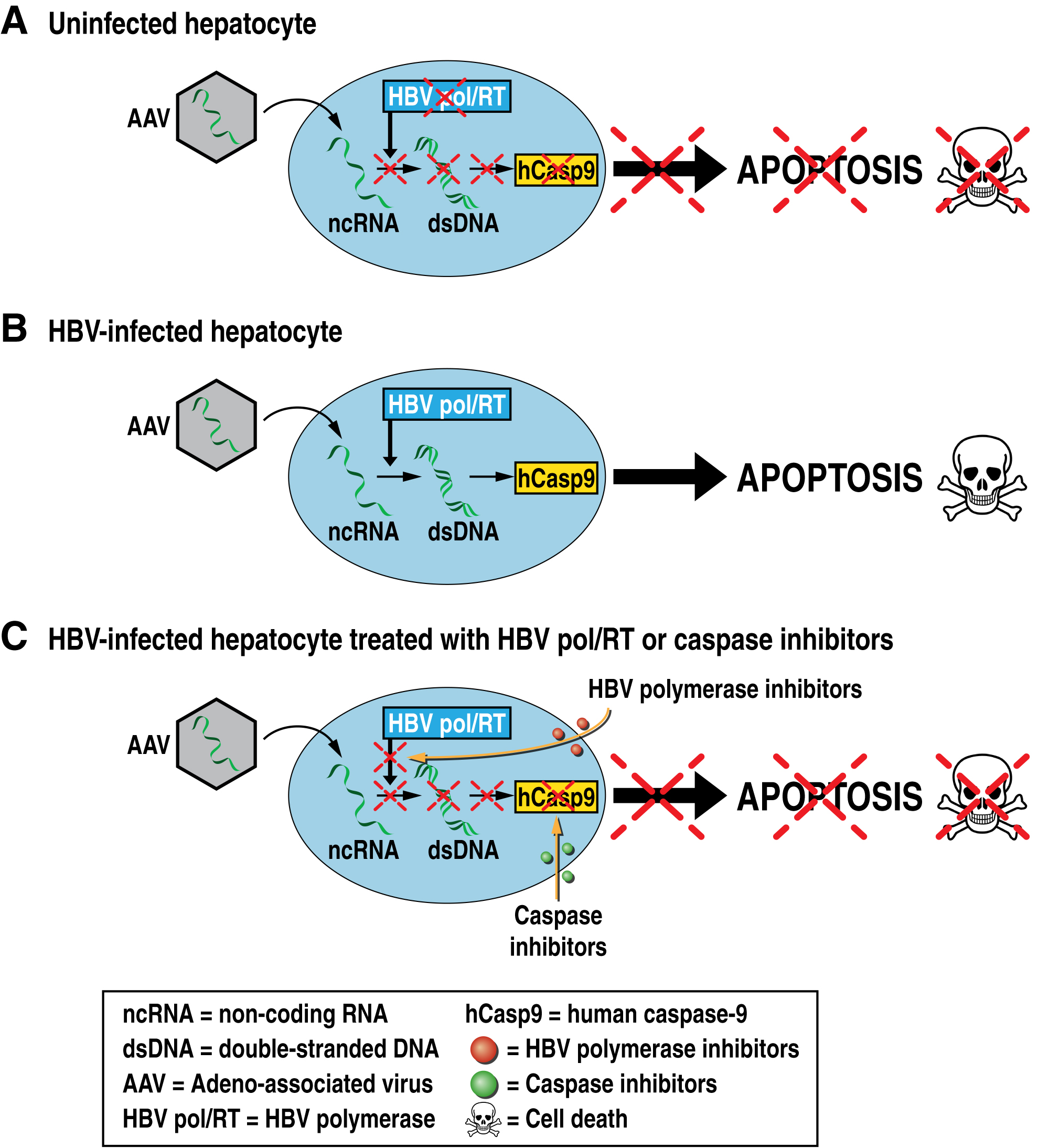
One strategy to cure HBV consists in the elimination of HBV by killing infected cells. Our lab is proposing a novel therapeutic approach for the elimination of HBV cellular reservoirs. We developed a new strategy to hijack the viral replication machinery to kill HBV-infected hepatocytes while preserving uninfected hepatocytes. We propose infecting HBV-infected hepatocytes with adeno-associated virus (AAV) vectors encoding the reverse complement opened reading frame (ORF) of genes of interest that will only be recognized and reverse transcribed by the HBV polymerase/reverse transcriptase present in infected cells, triggering the protein expression of the genes of interest. We developed AAV vectors, which deliver the reverse complement ORF of human caspase-9 (rc-hCasp9) – an initiator of the apoptosis caspase pathway – into infected livers that induces the killing of HBV-infected but not uninfected hepatocytes, leading to the elimination of HBV reservoirs without damaging liver functions.