Welcome to the Gallay Laboratory
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Therapies Overview
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the associated coronavirus disease 2019 (COVID-19) has precipitated a global pandemic heavily challenging our social behavior, economy, and healthcare infrastructure. The number of confirmed COVID-19 cases and deaths continues to rise rapidly: at the time of writing >4,400,000 deaths have been reported globally; ~14% of these in the U.S. where the number of confirmed cases has exceeded 38 million. U.S. COVID-19 deaths now exceed 630,000. Worldwide, recovery from this outbreak cannot begin until safe and effective therapeutic and prevention treatments are available. Although we now have several efficient vaccines against serious illness and death, there are no approved drugs, or immune therapeutics against SARS-CoV-2, and other prevention measures are required. The identification of new approaches to treat COVID-19 patients and prevent new infections is thus of utmost importance.
In collaboration with Starpharma (www.starpharma.com), we tested the anti-SARS-CoV-2 safety and efficacy of SPL7013, which is a large, highly branched, negatively charged dendrimer. We found that SPL7013 has broad spectrum antiviral and virucidal activity against SARS-CoV-2 (Paull et al., 2021a), including Variants of Concern (α, β, γ, and d) and Variant of Interest (κ), and other respiratory viruses including multiple subtypes of influenza A and human respiratory syncytial virus (HRSV) in vitro.
SPL7013 at 10 mg/mL incubated with virus and neutralized prior to infection resulted in >99.9% reduction in infectious virus compared with virus control within 30 seconds for the US and Alpha variants, and >99.99% within 30 seconds for the Delta variant in Vero E6 cells. Percent reductions of infectious virus achieved with 10 mg/mL SPL7013 are shown in the table below. Moreover, we demonstrated that the potent antiviral and virucidal activities of SPL7013 reduce SARS-CoV-2 replication and protect against pathogenic effects of infection in the K18-hACE2-mouse challenge model of infection (Paull et al., 2021b).
|
Virus:SPL7013† Incubation Time |
Percent Reduction of Infectious Virus vs Virus Control^ |
|||||
|
US |
Alpha |
Beta |
Gamma |
Delta |
Kappa |
|
|
30 seconds |
>99.9% |
>99.9% |
>99% |
>99% |
>99.99% |
>99.9% |
|
1 minute |
>99.9% |
>99.9% |
>99% |
>99% |
>99.99% |
>99.9% |
|
5 minutes |
>99.9% |
>99.99% |
>99.9% |
>99.9% |
>99.99% |
>99.9% |
|
15 minutes |
>99.99% |
>99.99% |
>99.99% |
>99.99% |
>99.999% |
>99.99% |
|
30 minutes |
>99.99% |
>99.99% |
>99.99% |
>99.99% |
>99.999% |
>99.99% |
† 10 mg/mL SPL7013; ^ virus without exposure to SPL7013
The negatively charged, branched, polyvalent structure of SPL7013 is presumed to act as a heparan sulfate mimetic. Heparan sulfate proteoglycans (HSPG) are ubiquitous structures, embedded in the cell membrane of essentially all epithelial cells. HSPG typically have a few negatively charged heparan sulfate chains attached to the transmembrane protein. Many viruses have adapted to use HSPG as a mechanism to attract virus towards, and bring them into association with, their specific cellular receptor. SARS-CoV-2 spike protein has three HSPG binding motifs (rich in positively charged amino acids) in areas essential for ACE2 binding and spike protein cleavage that are necessary for efficient viral entry into the cell. SPL7013 acts by electrostatically interfering with the functions of the external positively charged amino acids of the viral spike protein.
SPL7013 is included in a nasal spray as a barrier to trap and inactivate viruses, and reduce exposure to infectious viral load.
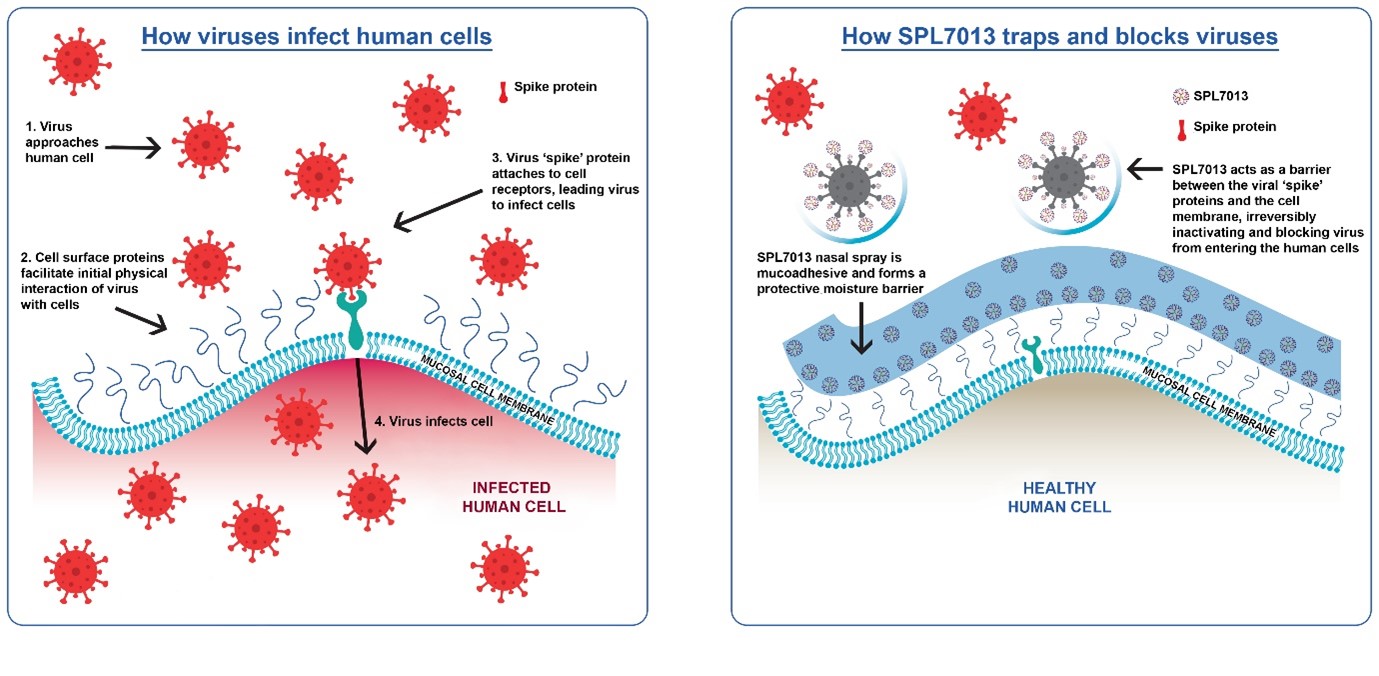
The spectrum of activity and mechanism of action of SPL7013 against the entry of SARS-CoV-2 and other respiratory viruses continues to be further characterized.
In addition, we are currently testing a panel of compounds with mechanisms of action distinct from SPL7013 in vitro as well as in vivo for their anti-SARS-CoV-2 and COVID-19 activities against all existing variants.
Paull JRA, Heery GP, Bobardt MD, Castellarnau A, Luscombe CA, Fairley JK, Gallay PA, Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. Antiviral Research. 2021; 191:105089 https://doi.org/10.1016/j.antiviral.2021.105089.
Paull JRA, Luscombe CA, Castellarnau A, Heery GP, Bobardt MD, Gallay PA. Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice. Viruses. 2021; 13(8):1656. https://doi.org/10.3390/v13081656

