Worm Institute for Research and Medicine
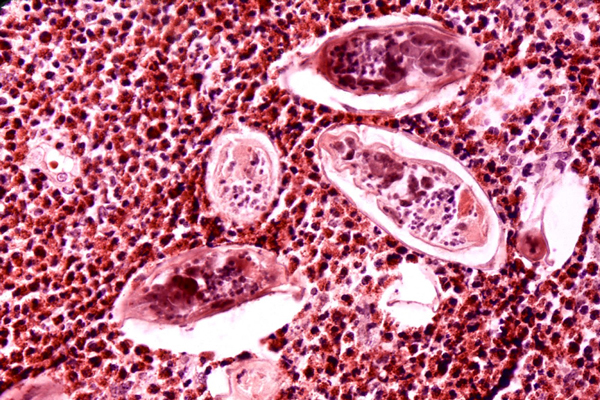
Worm diseases, while rare in the United States, afflict hundreds of millions of people in many parts of the world with painful, disfiguring, debilitating and often deadly diseases. The Worm Institute for Research and Medicine (WIRM), led by Dr. Kim D. Janda, was established in 2005 through a generous donation from philanthropist John J. Moores who had a long-time interest in worm-carried (filarial) conditions. In 1989, he had founded the River Blindness Foundation (later absorbed into The Carter Center of Atlanta where he had served on the Board of Trustees since its founding in 1994) to distribute a treatment for that disease in developing countries, mainly in sub-Saharan Africa. Mr. Moore’s gift to Scripps Research enabled our researchers to focus their work on worm-borne diseases and potential therapeutics for millions of people.
WIRM scientists have been investigating the basic science needed for the development of diagnostic tools for public health practitioners in the field to detect effectively and efficiently the presence of parasitic worms in a person's body. Our goal has been to translate our discoveries into unique approaches for the treatment of filarial infections throughout the world.
One of the topics WIRM investigators have tackled is the nematode, or worm, that causes onchocerciasis, one of the world's leading causes of blindness. Onchocerciasis is often referred to as "river blindness," because it occurs in areas close to fast flowing water where flies transmitting the parasite, a tiny worm called Onchocerca volvulus, like to lay their eggs. In severe cases, the worms cause lesions and massive inflammation in the eyes of the infected person, leading to vision problems and blindness. According to the Carter Center, approximately 20.9 million people are infected with the parasite that causes onchocerciasis, with more than 240 million at risk of the disease in sub-Saharan Africa, Latin America, and Yemen. River blindness can be treated effectively with the drug ivermectin that has been used for three decades now. However, there is still a need to find ways to detect the worms in the field to help public health efforts curtail new infections.
In addition to O. volvulus, WIRM researchers have been targeting several other organisms including:
- Brugia-malayi, Mansonella streptocerca and Wuchereria bancrofti — three threadlike worms that infect some 120 million people worldwide. These parasites lodge in lymphatic tissue and cause a disease known as lymphatic filariasis, a debilitating and disfiguring illness that causes elephantiasis, a disease characterized by severe swelling in the limbs and genitals.
- Dracunculis medinensis — a worm spread through unclean water that can grow to be several feet long in the body and causes the painful disease dracunculiasis, or Guinea worm disease.
- Schistosoma mansoni — a worm carried by freshwater snails, which causes the disease schistosomiasis, affecting some 200 million people worldwide.
- Dirofilaria immitis — a heartworm spread by mosquitoes that infects dogs and is common in the United States.
In order to develop more effective diagnostic tools for these diseases as well as more efficient treatments, a thorough understanding of the individual nematodes is required. However, as those afflicted with these ailments are primarily found in developing countries, the greater scientific community has not given these diseases much attention. WIRM aims to fill this gap and tackle some of the most challenging problems facing filarial parasitology today.
The filarial disease onchocerciasis, commonly referred to as “river blindness,” affects approximately 20.9 million people in Africa, Central and South America, and Yemen, with more than 240 million at risk, and is the second most common cause of preventable blindness in sub-Saharan Africa. Approximately 99 percent of O. volvulus infections occur in 31 countries in Africa with the remaining 1 percent found in six countries of the Americas, and Yemen on the Arabian Peninsula (Figure 1).

Symptoms of the disease include acute dermatitis and blindness, the result of which is the loss of 1 million disability-adjusted life years (DALYs) annually. The causative agent, the filarial nematode Onchocerca volvulus, is transmitted in its larval stage between human hosts through the bite of a Simulium (sp.) black fly (Figure 2).

Onchocerciasis and other neglected tropical diseases (NTDs) are generally classified as a group of medically disparate diseases afflicting the poorest people of the world's developing nations and resulting in acute illness, long-term disability and early death. Estimates attribute 12 of the NTDs together as causing 162,000 deaths annually with 14.8 million years lost to disability, causing a total burden of 19 million DALYs. Diagnostic tools currently in use for the detection of the NTDs are insufficient for measuring the extent of infection, making it difficult to distribute the appropriate therapies to those who need them. Within WIRM, we have applied mass spectrometric–based metabolomic technologies to the creation of diagnostic tests for identifying and classifying these diseases by measuring the blood of infected patients. In addition to serving a diagnostic purpose, relevant biomarkers could be a valuable means of understanding the complexity of pathogenic infection and may serve as chemical leads for therapeutic development.
Through onchocerciasis control programs in Africa (OCP 1974-2002; APOC, 1995-2015) and the Americas (OEPA, 1992-2012) conducting mass treatments with ivermectin, infection rates have been curbed. But the goal of eliminating and eradicating onchocerciasis, and neglected tropical diseases in general, remains and is contingent upon continued surveillance of the disease. It ultimately depends upon the ability to measure and track the progress of disease elimination and recrudescence. Thus, at WIRM we see advantages of a metabolomics-based diagnostic over onchocerciasis diagnostics currently implemented, including sensitivity, reproducibility, invasiveness, and the potential for multiplexing with biomarkers for other filarial and/or neglected tropical diseases.
In the discovery phase of WIRM’s research, the analysis of a set of 73 serum and plasma samples from African patients revealed a set of 14 biomarkers that showed excellent discrimination between Onchocerca volvulus–positive and -negative individuals by multivariate statistical analysis (Figure 3). Further analysis of these markers, applied to an additional sample set from onchocerciasis endemic areas where long-term ivermectin treatment has been successful, revealed that the biomarker set may also distinguish individuals with worms of compromised viability from those with active infection. Machine learning has allowed WIRM to extend the utility of the biomarker set from a complex multivariate analysis to a binary format applicable for adaptation to a field-based diagnostic, validating the use of complex data-mining tools applied to infectious disease biomarker discovery and diagnostic development.

Neglected tropical diseases (NTDs) affect over one billion people worldwide. Despite the gravity of such diseases on human health, the pharmaceutical industry has largely neglected the development of chemotherapies for NTDs. The massive deficit in global efforts stems mainly from the fact that these diseases affect poor people in poor regions of the world, and as such are not viewed as viable target markets for the pharmaceutical industry. However, more recently, many governmental, private sector and philanthropic organizations have begun to inject new funds into this area of research.
As part of this renewed effort in drug discovery for NTDs, a major goal of WIRM has been to identify potential therapeutic leads against new biological targets relevant to such diseases. Our efforts have focused on onchocerciasis, or “river blindness,” a leading cause of blindness in the developing world. Currently, the only drug available for mass treatment of onchocerciasis is ivermectin (Mectizan®, Merck). However, this drug is ineffective against adult worms and drug resistance appears to be emerging. Thus, there is a crucial need to identify new drug targets and agents that effectively treat onchocerciasis.
 Figure 1. Chitin metabolism.
Figure 1. Chitin metabolism.
As an untapped pathway with potential therapeutic relevance against O. volvulus, WIRM has focused on chitin metabolism. Chitin, one of the most widespread amino polysaccharides in nature, is a major structural component of arthropod exoskeletons, fungal cell walls, and the microfilarial sheath and eggshells of parasitic nematodes. However, it is entirely lacking from vertebrates. The dynamic biosynthesis and degradation of chitin is crucial for the growth and development of these organisms, and is regulated by two classes of enzymes, chitin synthases and chitinases (Figure 1). To date, only one chitinase from O. volvulus has been identified. OvCHT1 is expressed only in the infective L3 larvae and is stored within the granules of the cells of the esophageal glands until post-infective development, after which it is secreted and found mostly in the cuticle or outer body covering. Although its exact mechanism is not clear, it was hypothesized to likely play roles in host transmission, ecdysis (molting) and remodeling of the L4 cuticle and casting of the L3 cuticle. Because of the critical nature of these processes in the lifecycle of the parasite, WIRM believes that probing of the chitin metabolism with small, drug-like molecules should provide valuable insights for the future development of this biochemical pathway as a therapeutic target in O. volvulus.
Although chitinases, in general, have been implicated in several human disease pathways, and many highly complex natural product inhibitors have been reported, similar to NTDs, drug discovery efforts toward these targets are sparse. In the NTD field, current drug discovery strategies include piggy-back discovery (e.g., the screening of libraries that are already being assayed for a similar molecular target in another disease), de novo drug discovery and drug repositioning. Due to the time- and cost-effective nature of drug repositioning, WIRM has taken this approach for the discovery of new anti-onchocerciasis agents.

Figure 2. Structure of closantel and its impact on molting in O. volvulus. The ultrastructure images (in squares) were taken to determine what stage of molting was affected by closantel. Top square: Normal molting with complete separation of the L3 cuticle and the epicuticle of the newly developed L4 (separation between the black arrows). Bottom square: Treatment with closantel (100 μM) causes incomplete separation between the L3 cuticle and the L4 epicuticle (no free space between the black arrows).
We published our screening efforts against OvCHT1 in PNAS in 2010 (Gloeckner et al. PNAS 107, 3424). Using the Johns Hopkins Clinical Compound Library (JHCCL), a collection of 1,514 known drugs, as a source of drugs to be repositioned, a high-throughput fluorescence-based assay was employed to screen for inhibitors of OvCHT1. From these screening efforts, one drug was discovered with potent inhibition against OvCHT1, the known veterinary anthelmintic drug closantel, with an IC50 value of 1.6 ± 0.08 μM and a competitive inhibition constant (Ki) of 468 ± 84 nM (Figure 2). This compound was also found to be highly specific for filarial family 18 chitinases compared to those from protozoans and hCHTR. A significant finding was that closantel almost fully inhibited the L3 to L4 molt, and ultrastructural studies revealed an interesting closantel-induced phenotype in which the separation between the L3 cuticle and the newly formed L4 cuticle was inhibited and the cuticular material in between the cuticles was not fully degraded (Figure 2). Importantly, a similar phenotype has also been observed when L3 larvae were cultured with cysteine protease inhibitors or when the transcripts corresponding to O. volvulus cysteine proteases or a serine protease inhibitor were knocked down using RNA interference. It is WIRM’s hope that closantel’s negative impact on molting could lead to new strategies for targeting the progression of O. volvulus larvae to adult worms, which cannot be destroyed with current therapeutics.

Figure 3. Structure of a potent closantel fragment.
Closantel’s previously documented anthelmintic mode of action was thought to solely rely on its role as a proton ionophore, and its chitinase inhibitor activity was previously unknown, hence implicating a potential bimodal mechanism of action for its observed biochemical activity. With the newly discovered dual biochemical roles for closantel, WIRM initiated studies toward dissecting its activity-determining features. Using a “retro-fragment”-based approach, a compound was identified with a potency similar to closantel (IC50 of 5.8 ± 0.3 μM) (Figure 3). Using this lead fragment and its analogues, WIRM has been investigating the exact required molecular features underlying the proton ionophore activity as well as the chitinase inhibitory activity in an effort to map out which molecular fragments are required for individual and/or dual activities.
- Shirey RJ, Globisch D, Eubanks LM, Hixon MS, Janda KD. Noninvasive urine biomarker lateral flow immunoassay for monitoring active onchocerciasis. ACS Infect Dis. 2018;4(10):1423-31. PMID: 30141624.
- Globisch D, Eubanks LM, Shirey RJ, Pfarr KM, Wanji S, Debrah AY, Hoerauf A, Janda KD. Validation of onchocerciasis biomarker N-acetyltyramine-O-glucuronide (NATOG). Bioorg Med Chem Lett. 2017;27(15):3436-40. PMID: 28600214.
- Globisch D, Specht S, Pfarr KM, Eubanks LM, Hoerauf A, Janda KD. Litomosoides sigmodontis: A jird urine metabolome study. Bioorg Med Chem Lett. 2015;25(24):5804-7. PMID: 26573416.
- Gooyit M, Harris TL, Tricoche N, Javor S, Lustigman S, Janda KD. Onchocerca volvulus molting inhibitors identified through scaffold hopping. ACS Infect Dis. 2015;1(5):198-202. PMID: 27622649.
- Gooyit M, Tricoche N, Javor S, Lustigman S, Janda KD. Exploiting the polypharmacology of β-carbolines to disrupt volvulus molting. ACS Med Chem Lett. 2015;6(3):339-43. PMID: 25815157.
- Globisch D, Moreno AY, Hixon MS, Nunes AA, Denery JR, Specht S, Hoerauf A, Janda KD. Onchocerca volvulus-neurotransmitter tyramine is a biomarker for river blindness. Proc Natl Acad Sci U S A. 2013;110(11):4218-23. PMID: 23440222.
- Garner AL, Gloeckner C, Tricoche N, Zakhari JS, Samje M, Cho-Ngwa F, Lustigman S, Janda KD. Design, synthesis, and biological activities of closantel analogues: Structural promiscuity and its impact on Onchocerca volvulus. J Med Chem. 2011;54(11):3963-72. PMID: 21534605.
- Li Z, Garner AL, Gloeckner C, Janda KD, Carlow CK. Targeting the Wolbachia cell division protein FtsZ as a new approach for antifilarial therapy. PLoS Negl Trop Dis. 2011;5(11):e1411. PMID: 22140592.
- Denery JR, Nunes AA, Hixon MS, Dickerson TJ, Janda KD. Metabolomics-based discovery of diagnostic biomarkers for onchocerciasis. PLoS Negl Trop Dis. 2010;4(10):e834. PMID: 20957145.
- Eubanks LM, Silhar P, Salzameda NT, Zakhari JS, Xiaochuan F, Barbieri JT, Shoemaker CB, Hixon MS, Janda KD. Identification of a natural product antagonist against the botulinum neurotoxin light chain protease. ACS Med Chem Lett. 2010;1(6):268-72. PMID: 20959871.
- Gloeckner C, Garner AL, Mersha F, Oksov Y, Tricoche N, Eubanks LM, Lustigman S, Kaufmann GF, Janda KD. Repositioning of an existing drug for the neglected tropical disease onchocerciasis. Proc Natl Acad Sci U S A. 2010;107(8):3424-9. PMID: 20142509.
- Park J, Dickerson TJ, Janda KD. Major sperm protein as a diagnostic antigen for onchocerciasis. Bioorg Med Chem. 2008;16(15):7206-9. PMID: 18632276.
Related Links
| Janda Laboratory Website |
| Carter Center River Blindness |
| World Health Organization - Onchocerciasis |

