|
(page 2 of 2)
Once activated, T cells will proliferate and undergo a massive
expansion, differentiating into helper and killer T cells.
And once the T cells do what they are supposed to do and get
rid of the pathogen, they are eliminated—somehow cleared
from the system, except for a fraction of the cells.
"There is a great deal of interest in how these cells are
being destroyed," says Sprent. "We're interested in the signaling
molecules involved [with keeping them alive]."
What keeps these cells alive are signals that they receive
from their cell surface molecules, telling them to live. Some
cells survive and continue to circulate in the body in a resting
state. These cells are kept alive by the immune system and
are called memory cells, and these are able to mount a much
faster and more aggressive immune response to another challenge
with the same pathogen for which they are specific. Memory
cells as a population live almost indefinitely, dividing every
so often into daughter cells.
The researchers are trying to figure out the mechanisms
and key regulators involved in maintaining memory T cells,
with Sprent concentrating on killer T cells and Surh focusing
on helper T cells.
One signal that Sprent has already found is the chemokine
interleukin-15 (IL-15). Memory killer T cells are kept alive
by IL-15 contact, and if you take away IL-15, the memory cells
will die. What keeps memory helper cells alive is currently
unknown.
How T Cells Grow Up and How They Grow Old
The two scientists are also interested in how T cells develop
and live under normal conditions—as naïve T cells,
which is what scientists call mature T cells before they have
been activated. When T cells come out of the thymus, they
join a highly regulated pool of cells in the periphery. The
body maintains and regulates its pool of naïve T cells
and resting memory cells. This regulation is different from
the regulation that they are subjected to in the thymus, and
it is also different from the regulation to which previously
activated memory T cells are subjected.
"It used to be thought that once T cells develop in the
thymus and come out to peripheral tissues [as mature cells],
they just sit and do nothing—just wait for an antigen.
That doesn't seem to be true," says Surh.
In fact, the fate of T cells that have not been activated
seems to be predetermined during their development in the
thymus. What happens in the thymus is crucial for what happens
for the rest of the life of the T cell, whether it expands
or dies.
"Whatever they learned there seems to determine how they
will behave [in the periphery]," says Surh.
Sprent and Surh have developed some models that maintain
a larger pool of T cells, and are now trying to discern whether
the larger pool results from an increased production of T
cells in the thymus or from an expansion of naïve T cells
in the periphery.
The body has a homeostatic mechanism that maintains the
pool of naïve T cells—a mechanism that takes no
cues from the environment. This homeostasis determines the
number and kind of naïve T cells that are kept in circulation.
If T cell numbers drop to low level, the body senses this
and tells remaining naïve T cells to undergo spontaneous
expansion and fill up the body again.
This can be demonstrated quite dramatically by injecting
T cells into in vivo models that have no T cells. The newly
injected T cells recognize the body's need for T cells, and
as a consequence, they begin to expand. Injecting the same
T cells into a normal body does not result in expansion.
naïve T cells need at least one chemical signal to
maintain their population. The cytokine IL-7, a growth factor
protein that circulates systemically, seems to be important
for determining how many T cells are kept around. In laboratory
models, Surh has observed that increasing IL-7 causes T cells
to expand, and removing T cells causes IL-7 levels to increase,
which in turn instructs the remaining T cells to expand, preparing
the body to fend off the inevitable challenges from foreign
pathogens.
1 | 2 |

|
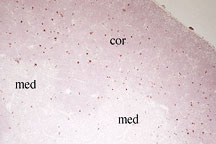
Visualization of dying cells in the
normal mouse thymus. Thymocytes undergoing apoptosis (red)
are widely distributed throughout the cortex (med), whereas
they are less frequent in the medulla (med). This and other
data indicate that most of cell death in the thymus is due
to lack of positive selection rather than from negative selection.
(Apoptotic cells are stained using the TUNEL technique.)
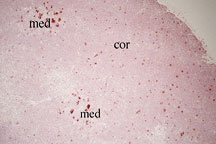
Visualization of negative selection.
Apoptotic cells (red) are depicted in a situation where nearly
all the positive selected cells are designed to undergo negative
selection. Thus, in addition to the background apoptotic cells
in the cortex (cor), large clumps of dying cells are visible
in the medulla (med). (TUNEL staining was performed on TCR
V beta 5 transgenic mice in an H2-E+ background where endogenous
mammary tumor viral (MTV) antigens mediate negative selection
of transgenic thymocytes. )
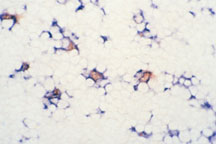
Rapid clearance of apoptotic cells by
resident macrophages. Nearly all apoptotic cells (red) present
in the cortex of normal mouse thymus are found inside resident
macrophages (blue) indicating that apoptotic cells are rapidly
engulfed by nearly phagocytic cells. (Normal mouse thymus
was double stained by the TUNEL technique and an antibody
to macrophages.)
|

