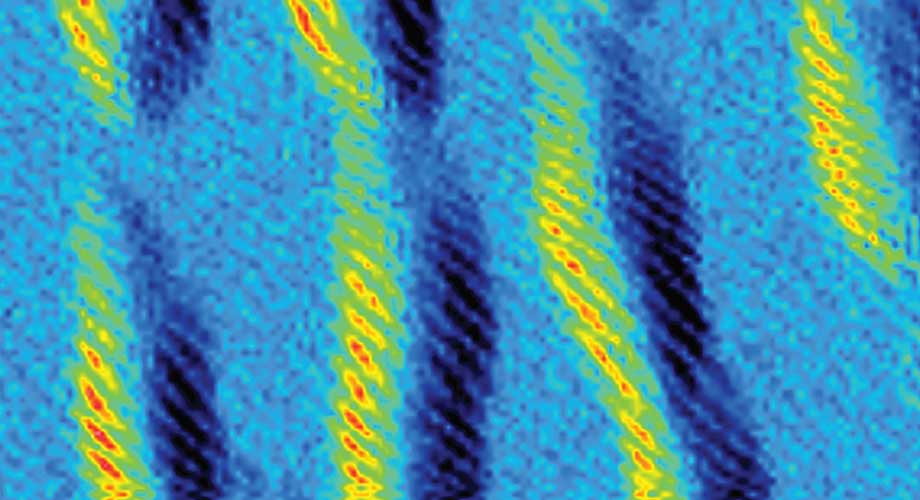
By harnessing click chemistry, researchers were able to build SOF4-based polymers, indicated by these bright helical coils. This image was taken by atomic force microscopy. Image credit: Han Zuilhof lab, Wageningen University.
Polymers “click” together using green chemistry
With applications in drug discovery and materials science, a new library of polymers can be generated in in an environmentally safe way.
August 16, 2021
LA JOLLA, CA—Some of the biggest roadblocks in chemistry are the highly reactive nature of molecules and their difficulty in preparation. Such is the case for the sulfur-based gas SOF4, discovered over a century ago. Now, a collaboration of chemists has discovered a novel way to use the molecule safely as a building block for a variety of new biochemical products.
In a team effort that includes Scripps Research’s Nobel laureate K. Barry Sharpless, PhD, and associate professor Peng Wu, PhD, as well scientists from Cold Spring Harbor Laboratory (CSHL) and Wageningen University, the research describes a new set of modifiable polymers (chemicals consisting of repeating units) made from SOF4. The findings appear in Nature Chemistry.
Before molecules could be stitched together into polymers, the team first needed access to sufficient levels of the SOF4 gas. “There was no more SOF4 available in the world,” says Suhua Li, PhD, lead author of the study and a former postdoc in Sharpless’ lab. “The great potential of the SOF4 chemistry inspired me to make the gas by myself, even though the procedure seemed dangerous.”
The SOF4 molecule could then be used as a hub to link together diverse components into a modular family of new and potentially valuable materials. The scientists harnessed click chemistry to conduct these reactions in an environmentally safe way without dangerous solvents and polluting byproducts. Click chemistry is a method of synthesizing larger molecules by combining smaller chemicals together, similar to how LEGO® bricks click together, using very reliable and fast reaction chemistry.
By forming these long chain polymers – with room for important modifications – the team is amassing a vast new library of molecules, each with its own distinct properties and potential applications in drug discovery and material science. “It is the making and breaking of the nascent, growing polymer links that let us access what seems magical,” says Sharpless.
The study, “SuFExable polymers with helical structures derived from thionyl tetrafluoride,” was supported by the National Science Foundation, National Institutes of Health, Australian Research Council for Supporting Future Fellowship, Guangdong Natural Science Funds for Distinguished Young Scholar, Program for Guangdong Introducing Innovative and Entrepreneurial Teams, Pearl River Talent Recruitment Program, National Science Foundation of China, King Abdulaziz University, Office of Science, Office of Basic Energy Sciences of the US Department of Energy.
Authors include Suhua Li, Gencheng Li, Bing Gao, Sidharam P. Pujari, Xiaoyan Chen, Hyunseok Kim, Feng Zhou, Liana M. Klivansky, Yi Liu, Hafedh Driss, Dong-Dong Liang, Jianmei Lu, Peng Wu, Han Zuilhof, John Moses and K. Barry Sharpless.
For more information, contact press@scripps.edu

