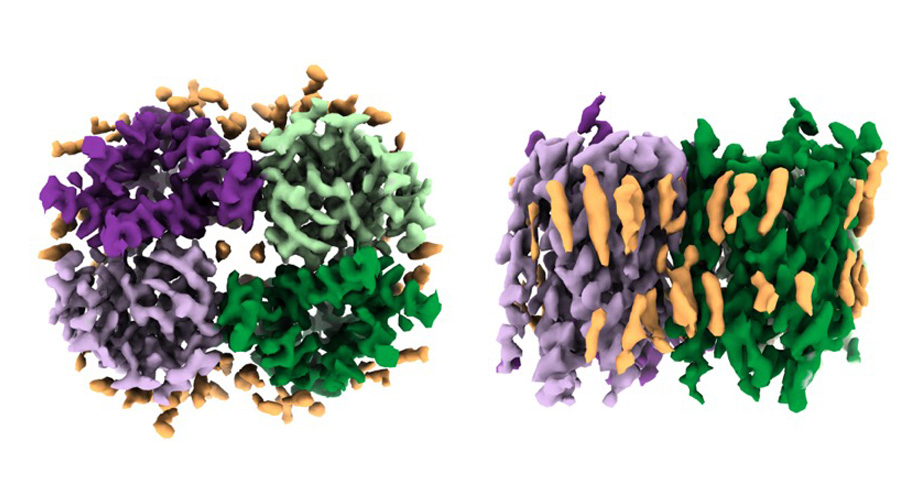
Top and side views of cryo-EM density map of the Otopetrin 3 (Otop3) orthologue from chicken. The homodimeric structure (green and purple) of Otop3 is surrounded by lipids and cholesterols (yellow).
Scripps Research scientists reveal exceptional structural detail of novel protein family involved in sour taste reception and prevalent in digestive and immune systems
Using cryogenic electron microscopy, researchers gained crucial structural and functional insights into proton channels involved in balance, taste and yet-unidentified roles in human health.
June 03, 2019
Recently discovered proteins that play a key role in inner-ear balance and our ability to taste sour foods may hold many other important roles in human health, as they are prevalent in the digestive tract and immune system.
In a new study that provides an unprecedented level of structural detail for these proteins, known as otopetrins, Scripps Research scientists—in collaboration with scientists at the University of Southern California and Oxford University—have opened the door to finding new mechanisms for potentially treating a wide range of medical conditions. The study appears in Nature Structural & Molecular Biology.
“Despite their broad roles in biology, our understanding of otopetrins is only beginning to unfold, and I am certain they play critical roles in our health that have yet to be discovered,” says Andrew Ward, PhD, a Scripps Research professor in the department of Integrative Structural and Computational Biology. “Our structural work represents a very early foundation for understanding how these unique proteins accomplish their physiological functions.”
Otopetrins are among a class of proteins known as ion channels, which selectively allow small molecules called ions to pass through a cell’s outer membrane. Otopetrins are unique in that they allow hydrogen ions to cross the membrane, whereas other ion channels allow sodium or potassium to cross. Among their many physiological roles, these channels regulate cellular pH—a measure of acidity or alkalinity.
In seminal research published in Science in January 2018 out of University of Southern California, Emily Liman, PhD, professor of Biological Sciences, and graduate student Yu-Hsiang Tu showed that otopetrins function as proton channels, which are a subset of ion channels, and uncovered their likely role in sensing acids as part of sour taste perception.
However, because otopetrins are highly expressed throughout the body, including in the digestive system, it’s possible they may offer a route to treat conditions such as inflammatory bowel disease.
With the goal of gleaning new insights into how these channels function, Liman reached out to Ward to collaborate on the latest project, knowing of Ward’s past successes in solving the structures of other novel ion channels. Liman’s lab cloned additional species variants of otopetrins and conducted functional studies to support Ward’s structural work.
The study also involved collaboration with biophysicist Mark Sansom’s team at the University of Oxford, which used molecular simulations to discern how cholesterol and water molecules interact dynamically with otopetrins. The simulations were conducted by Che Chun (Alex) Tsui, a Skaggs-Oxford graduate fellow, working between the Ward and Sansom labs.
To conduct their research, Ward and his team used state-of-the-art imaging technology called cryogenic electron microscopy (cryo-EM) to characterize otopetrin structures from zebrafish and chicken. This allowed them to see that the assembled protein is formed of two identical subunits, and to start to resolve how ions could move through the channel. “We were blown away to see the structures, coming so soon after identifying their function,” Liman says.
“It was exciting to be able to resolve the structures in nanodiscs, which impart a native-like membrane-lipid environment to the protein,” said Kei Saotome, PhD, research associate in Ward’s lab at Scripps Research and first author of the paper. “This allowed us to resolve an exceptional number of protein-lipid interactions that are likely important for function.”
Protein-lipid interactions are an increasingly hot topic in membrane protein research, and the combination of cryo-EM and molecular simulations allows these to be probed in detail.
Now that the team has defined the structure of the proton channels, it plans to probe the structure at even higher resolution to observe water molecules and ions that are critical for function but typically very difficult to observe. Because otopetrins are one of only two known mammalian proton channels, the growing body of research will present an opportunity to understand the molecular basis of a fundamental function in cells.
Ward’s team also hopes to eventually move on from using otopetrin protein channels from zebrafish and chicken and begin conducting research with actual human samples. “This would most impactful for studying human health and disease,” Saotome says.
In addition to Ward and Saotome, authors of the study, “Structures of the Otopetrin Proton Channels Otop1 and Otop3,” include Bochuan Teng of USC; Che Chun (Alex) Tsui of University of Oxford and Scripps Research; Wen-Hsin Lee of Scripps Research; Joshua P. Kaplan of USC, Yu-Hsiang Tu of USC, Mark S. P. Sansom of University of Oxford; and Emily R. Liman of USC.
This work was supported by the Ray Thomas Edwards Foundation, the National Institutes of Health [NIDCD013741], Wellcome [208361/Z/17/Z], BBSRC [BB/N000145/1 and BB/R00126X/1), and EPSRC [EP/R004722/1]; Jane Coffin Childs Memorial Fund for Medical Research; the Skaggs-Oxford Scholarship; and Croucher Foundation.
For more information, contact press@scripps.edu

