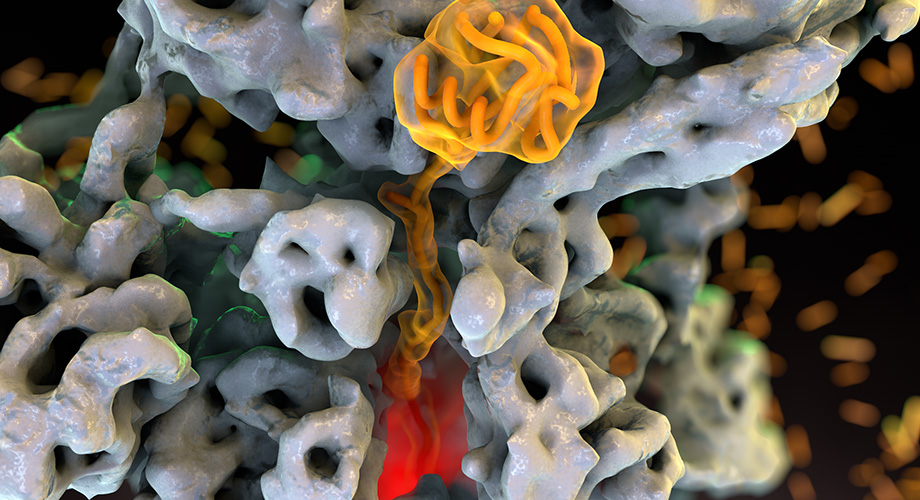
The Scripps Research scientists and their collaborators used cryo-EM to visualize the proteasome, a cellular machine that destroys proteins that are no longer needed or endanger the cell. Credit: Lander lab.
Biologists show inner workings of cellular ‘undertaker’
December 04, 2018
LA JOLLA, CA – One of a cell’s most important responsibilities is to break down and recycle proteins that are no longer needed or endanger the cell. This task is carried out by a cellular nanomachine called the proteasome.
Scientists from Scripps Research deciphered how the proteasome converts energy into mechanical motion that untangles and unfolds proteins for destruction. The findings, published in Science , could help us understand how proteasomes keep diseases such as Parkinson’s and Alzheimer’s at bay.
“The proteasome is like the cellular undertaker for proteins that are no longer needed or pose a threat to the cell’s health,” says senior author Gabriel Lander, PhD, an associate professor on Scripps Research’s California campus. “As we age, our proteasomes become less efficient, which can lead to a variety of diseases.”

Gabriel Lander, PhD, and Andres Hernandez de la Peña, PhD, of Scripps Research. Credit: Scripps Research.
Lander’s lab focused on visualizing the inner workings of the proteasome for over five years. Much of this work was done in collaboration with Andreas Martin at the University of California, Berkeley, who was co-senior author of the study. The proteasome does not readily reveal its secrets and has been a challenging biological target. Although it’s one of the largest machines in the cell, the proteasome is nonetheless incredibly small. “Imagine looking up at the moon and trying to read a street sign placed on its surface – that’s the scale we’re dealing with,” says Lander. To further complicate matters, the proteasome’s structure is very complex, containing a motor and many moving parts.
The researchers were able to visualize the proteasome’s inner workings thanks to a structural methodology called cryo-electron microscopy (cryo-EM). This technology freezes biological complexes in mid-motion, allowing scientists to see many different conformations at the same time.
“It’s like taking a snapshot of a busy freeway at rush hour. The engines powering the cars will all be in different states of firing, but by looking at millions of them we can figure out precisely how they work,” says Andres Hernandez de la Peña, PhD, a postdoctoral fellow in Lander’s lab and the paper’s first author. “To truly understand how a motor works, you need to observe it while it’s running. Other structural methods would require us to shut down the motor or throw a wrench into it to stall it. Cryo-EM allowed us to catch the proteasome red-handed, with pistons pumping as it pulled on a targeted protein for degradation.”
Specifically, the researchers learned how ATP, the fuel source of the cell, powers movements within the proteasome’s motor that enable it to pull in proteins through its central channel. Like pulling a tangled ball of thread through the eye of a needle, the proteasome properly positions proteins, threads them, and feeds them as a single unfolded strand to the degradation chamber.
Now that the scientists have a better understanding of how the proteasome works, there are significant ramifications for studying neurogenerative diseases. “This has implications for understanding what happens when proteasomes encounter proteins that are very tightly folded, such as the amyloid proteins associated with Alzheimer’s disease,” Lander says.
“This is also important for cancer therapy,” Hernandez adds. Some drugs that are used to treat the blood cancer multiple myeloma are targeted against the proteasome. “These drugs target the shredder blades rather than the motor,” he adds. “Now that we understand the motor, we can work to develop a more regulated response and target similar motors found in other cellular machines.”
“Thanks to this research we’ve become much better mechanics,” Lander concludes. “Going forward we’ll have a much better shot at figuring out what’s happening to our proteasomes as we age, and work out how to keep them running efficiently and effectively, like well-oiled machines.”
This study, “Substrate-Engaged 26S Proteasome Structures Reveal Mechanisms for ATP-Hydrolysis-Driven Translocation,” was funded by National Institutes of Health (grants DP2EB020402 and R01-GM094497), the American Cancer Society (grant 132279-PF-18-189-01-DMC), and the Howard Hughes Medical Institute.
For more information, contact press@scripps.edu

