Scientists design antibody-studded cells to destroy cancer
December 07, 2018
LA JOLLA, CA – Scripps Research scientists have developed a quick, cheap way to attach entire antibodies to living cells.
Study leader Peng Wu, PhD, an associate professor at Scripps Research, says the attached antibodies work like a GPS, guiding immune cells to kill tumor cells. In fact, the researchers have used this new method to locate and destroy aggressive breast cancer cells in a mouse model.
“We think this can be quickly translated from the lab bench to the clinic,” says Wu, senior author of the new study, published December 7, 2018 in ACS Central Science.
Cancer immunotherapy is a growing field, and scientists today are testing several strategies to genetically engineer cells or to modify the cell surface to better target cancer cells. At the moment, these methods can be extremely expensive and can interfere with cell function.
The advantage of the new Scripps Research method is that the chemical reaction can be done with FDA-approved materials, in one step, in a matter of minutes.
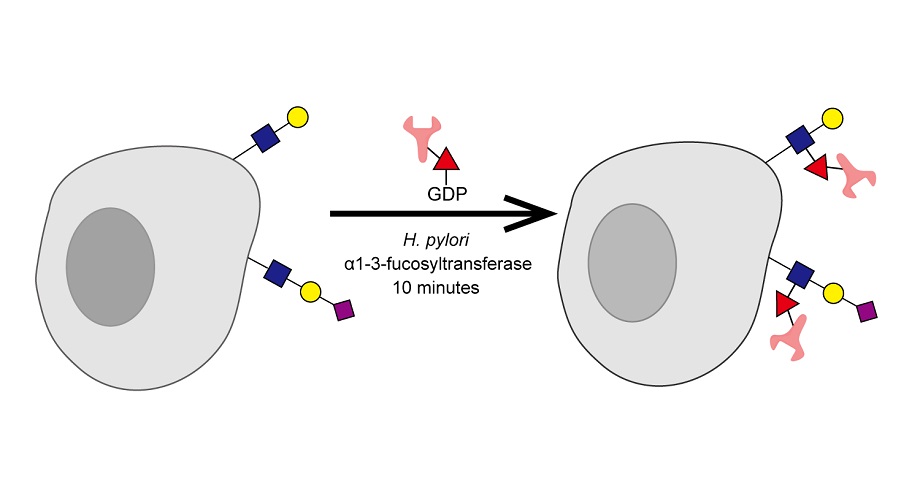
The key player is an enzyme called fucosyltransferase, which normally transfers a sugar molecule called fucose from the GDP-fucose donor to another sugar in the body. Wu and his colleagues discovered that this enzyme, originally isolated from Helicobacter pylori in the human stomach, can even transfer antibodies that are linked to GDP-fucose to the cell surface sugar molecules.
Wu, who has been studying this enzyme for 10 years, says it was a shock that the method worked—since antibodies are much heavier than sugar molecules. “This is a gigantic thing to transfer,” says Wu.
The researchers used this method to construct “antibody-cell conjugates.” Antibodies are important to these conjugates because they naturally seek out specific targets—such as unique molecules on tumor cells. The next step was to test whether antibody-cell conjugates could work in cancer immunotherapy.
For their cells, the researchers used immune cells called natural killer cells (NK-92 line). Although these cells are currently undergoing clinical trials for use against cancer cells, they aren’t naturally drawn to any specific targets. The researchers used their method to add an FDA-approved monoclonal antibody called Herceptin to target HER2+ breast cancer cells. These antibody-cell conjugates proved effective at significantly suppressing tumor growth in a mouse model.
Although more tests need to be done, Wu says the method holds promise for fighting human cancers. He is encouraged by the fact that that Herceptin is already FDA-approved and NK-92 cells have already been approved for use in clinical trials.
Importantly, NK-92 cells aren’t patient-specific, meaning they can be produced in bulk and potentially given to any patient. Wu sees this new “chemoenzymatic” approach as a useful complement to CAR-T cell therapies, in which cells from a patient’s own body are reprogrammed to target a protein in cancer cells.
A follow-up experiment showed that antibody-cell conjugates could also help the immune system’s CD8+ T cells to better target tumor cells.
Going forward, the team plans to expand the applications for their method. “Using this method, we can transfer anything: nanoparticles, small molecule drugs, anything,” says study co-first author Jie Li, PhD, a research associate at Scripps Research. “And we can do that with a single step.”
The researchers are also looking forward to collaborating with other labs at Scripps Research, including the lab of Richard Lerner, MD, who has developed an extensive library of antibodies that could be used in the new reaction.
Additional authors of the study, “A Single-step Chemoenzymatic Reaction for the Construction of Antibody-cell Conjugates,” were Mingkuan Chen (co-first author), Zilei Liu, Linda Zhang and Brunie H. Felding of Scripps Research; Kelley W. Moremen of the University of Georgia; Gregoire Lauvau of the Albert Einstein College of Medicine; and Michael Abadier and Klaus Ley of the La Jolla Institute for Immunology.
The research was supported by the National Institutes of Health (grants GM113046, GM093282 and GM103390).
For more information, contact press@scripps.edu

