The Leman Lab
RESEARCH
We use a combination of chemical synthesis, biophysical techniques, molecular biology, and biochemistry to develop peptide-based materials. Current areas of research include synthetic lipopeptide nanoparticles as high-density lipoprotein (HDL)-mimetics, sequence-adaptive dynamic nucleic acid analogs, and prebiotic chemistry.
Our ultimate goal is to better understand biology and treat disease by advancing the basic understanding of living chemical processes and the origins of complex chemical networks.
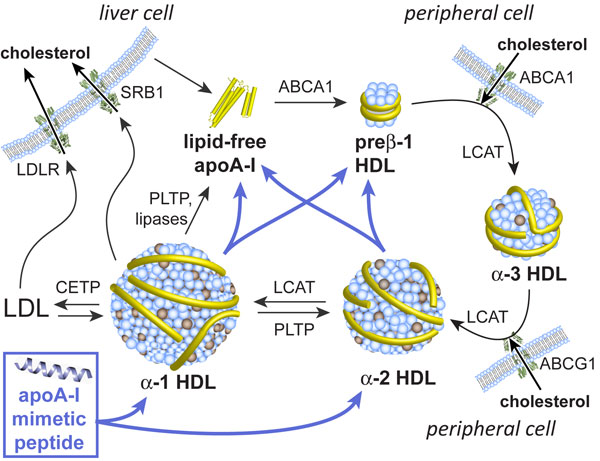
Multivalent apolipoprotein mimetics
Atherosclerosis is a causative factor in nearly one third of all deaths in the United States. A promising strategy for combating atherosclerosis is by enhancing the function of high-density lipoproteins (HDLs, "good cholesterol"), which remove excess cholesterol from peripheral tissues for delivery to the liver and elimination.
We have developed a new class of peptides that enhance the function of HDLs both in vitro and in vivo by mimicking apolipoprotein A-I (apoA-I), the major protein component of HDL. ApoA-I plays a key role in the function of HDL and its anti-atherogenic properties have been documented in numerous studies, including human clinical trials. However, the high production costs and lack of oral bioavailability of apoA-I have made it impractical for chronic use in the management of atherosclerosis. Our apoA-I mimetics, in the presence of lipids, can be fashioned into discoidal HDL-like nanolipopeptide constructs. The constructs interact with and remodel the plasma HDL network, increase the level of pre-beta HDL particles (the subspecies of HDLs thought to be the most anti-atherogenic), and enhance cholesterol efflux from macrophage cells. By forming HDL-like nanoparticles, apoA-I mimetics may also increase the overall flux of cholesterol transport by serving as additional plasma lipid carriers. Moreover, the peptides prevent the development of aortic lesions over 10 weeks in LDLr-/- mice (an animal model of atherosclerosis that most resembles hyperlipidemia in humans). Surprisingly, the apoA-I mimetics exhibit high oral bioavailability in mice, thus providing a promising path toward the development of future therapeutic agents.
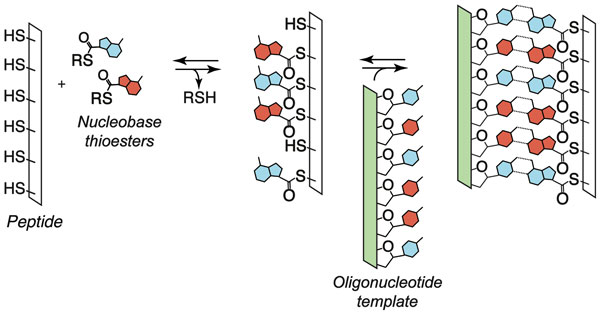
Dynamic sequence-adaptive peptide nucleic acids
Recently, we have been exploring the hypothesis that the dynamic nature of oligomers assembled by reversible reactions could have imbued them with advantageous features in the context of chemical evolution. Such molecules assemble, disassemble, and change their sequences under the driving force of thermodynamics. We designed and characterized a sequence-adaptive peptide nucleic acid that efficiently self-assembles via reversible covalent anchoring of nucleobase recognition units onto simple oligo-dipeptide backbones (thioester peptide nucleic acids, tPNA) and undergoes dynamic sequence modification in response to changing templates in solution. The oligomers specifically self-pair with complementary tPNA strands and cross-pair with RNA and DNA in Watson-Crick fashion. Thus, tPNA combines base-pairing interactions with the side chain functionalities of typical peptides and proteins. These characteristics might prove advantageous for the design or selection of catalytic constructs or biomaterials that are capable of dynamic sequence repair and adaptation.
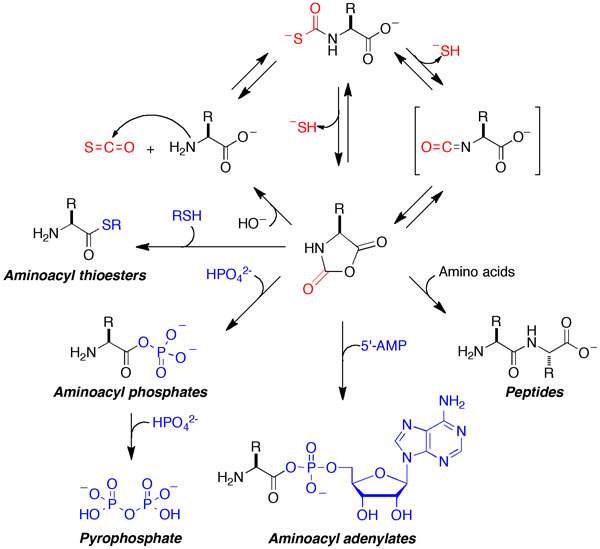
Prebiotic chemistry
Understanding how the chemical process of life originated on the ancient Earth is an intriguing question. Many of the most important molecules in biology– proteins, DNA, RNA, and carbohydrates– are polymers. Unfortunately, attempts to demonstrate plausibly prebiotic polymerization reactions have met with limited success. The polymerization agents that seem plausible as prebiotic molecules are few in number, and relatively inefficient.
Our lab showed that carbonyl sulfide (COS), a simple gas that is present in the emissions from present-day volcanoes, is a condensing agent that brings about the polymerization of amino acids to form peptides under mild conditions in aqueous solution. Depending on the reaction conditions employed, exposure of amino acids to COS generated peptides in yields of a few percent up to 60% in minutes to hours at room temperature. We further investigated the reactions of COS in the context of phosphate activation reactions, which are involved in the polymerization of DNA and RNA. We found that COS is an effective reagent for the synthesis of aminoacyl phosphates and aminoacyl adenylates. We further showed that these species, in the presence of certain divalent ions, are phosphorylating agents. These amino acid-dependent activations of phosphate derivatives, which occur under mild aqueous conditions in parallel with the production of peptides, suggest that peptide synthesis and phosphoryl transfer reactions might have been coupled on the prebiotic Earth.