The Gallay Laboratory
Projects
| Click here for the Hepatitis C Project HIV Attachment Receptor Agonists and Antagonists as Microbicidal Candidates Given that cell-free virus ineffectively crosses the genital epithelium in the absence of lesion, it is likely that HIV hijacks host cells as Trojan horses to cross the normally impermeable genital epithelium. It has been postulated that HIV exploits Langerhans and dendritic cells to facilitate its safe passage through the genital epithelium (Figure 1). We obtained several lines of evidence suggesting that HIV exploits specific receptors called syndecans and lectins to attach to the surface of the genital epithelium, Langerhans and dendritic cells. Syndecans and lectins thus represent new targets for the development of topical microbicides. We developed compounds that prevent both HIV-syndecan and HIV-lectin interactions. Specifically, we developed syndecan and lectin antagonists that neutralize the syndecans and lectins expressed on the mucosal surface and on the surface of Langerhans and dendritic cells. We also developed syndecan and lectin agonists that neutralize the syndecan- and lectin-binding site within HIV, the viral glycoprotein gp120. 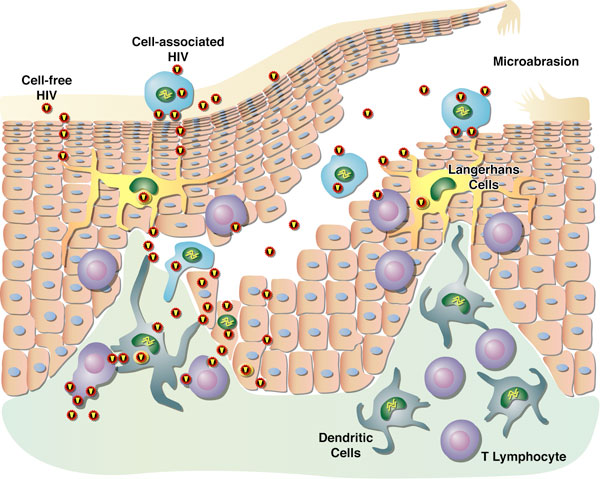 Figure 1. Model for cell-free and cell-associated HIV transmission through the genital epithelium.We demonstrated that syndecan antagonists and agonists efficiently prevent the passage of HIV through primary human genital epithelial cells (Figure 2), further supporting the notion that syndecans represent attractive targets for the development of microbicides. Since compounds that interrupt gp120-syndecan interactions have never been exploited as microbicides, they represent a novel class of microbicides and thus differ from existing tools. One advantage of using compounds, which target gp120-syndecan interactions as microbicides, is that all primary R5, X4 and R5X4 HIV as well as HIV-2 isolates bind syndecans. Thus, these compounds will have a broader impact than, for example, RANTES derivatives, which neutralize R5 viruses only. Since we found that compounds that block gp120-syndecan interactions also block gp120-CCR5 interactions, their dual inhibitory effects represent another advantage of using them as microbicides. Moreover, another advantage of using microbicides that target syndecans is that many sexually transmitted pathogens also exploit syndecans for host colonization such as herpes simplex virus and Neisseria gonorrhoeae. Thus, microbicides targeting gp120-syndecan interactions will exhibit a broad inhibitory spectrum against sexually transmitted pathogens. Importantly, we also found that syndecan antagonists and agonists as well as lectin antagonists and agonists prevent the contact between HIV and Langerhans and dendritic cells. Altogether these promising results suggest that we possess tools, which could serve as microbicidals in order to interrupt HIV transmission in humans. These HIV attachment receptor agonists and antagonists are currently tested in macaques in an HIV vaginal transmission model. The in vivo approach should not only determine whether these inhibitors represent new microbicidal candidates, it also should allow us to assess the contribution of Langerhans and dendritic cells to the initial steps of HIV transmission. Figure 1. Model for cell-free and cell-associated HIV transmission through the genital epithelium.We demonstrated that syndecan antagonists and agonists efficiently prevent the passage of HIV through primary human genital epithelial cells (Figure 2), further supporting the notion that syndecans represent attractive targets for the development of microbicides. Since compounds that interrupt gp120-syndecan interactions have never been exploited as microbicides, they represent a novel class of microbicides and thus differ from existing tools. One advantage of using compounds, which target gp120-syndecan interactions as microbicides, is that all primary R5, X4 and R5X4 HIV as well as HIV-2 isolates bind syndecans. Thus, these compounds will have a broader impact than, for example, RANTES derivatives, which neutralize R5 viruses only. Since we found that compounds that block gp120-syndecan interactions also block gp120-CCR5 interactions, their dual inhibitory effects represent another advantage of using them as microbicides. Moreover, another advantage of using microbicides that target syndecans is that many sexually transmitted pathogens also exploit syndecans for host colonization such as herpes simplex virus and Neisseria gonorrhoeae. Thus, microbicides targeting gp120-syndecan interactions will exhibit a broad inhibitory spectrum against sexually transmitted pathogens. Importantly, we also found that syndecan antagonists and agonists as well as lectin antagonists and agonists prevent the contact between HIV and Langerhans and dendritic cells. Altogether these promising results suggest that we possess tools, which could serve as microbicidals in order to interrupt HIV transmission in humans. These HIV attachment receptor agonists and antagonists are currently tested in macaques in an HIV vaginal transmission model. The in vivo approach should not only determine whether these inhibitors represent new microbicidal candidates, it also should allow us to assess the contribution of Langerhans and dendritic cells to the initial steps of HIV transmission.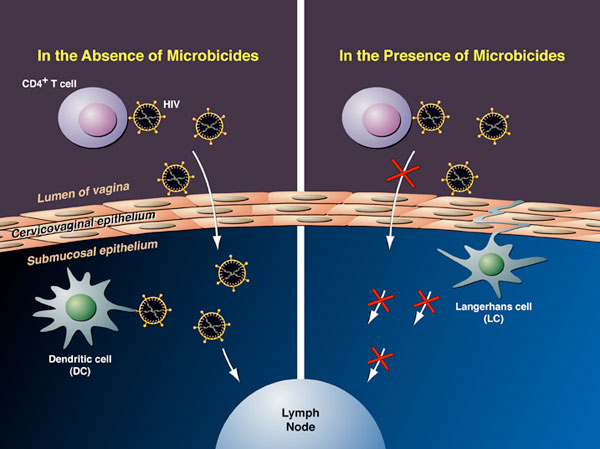 Figure 2. Model for the Antiviral Activities of Syndecan- and Lectin-Agonists and AntagonistsPreclinical Development of Anti-HIV Microbicidal Peptides Figure 2. Model for the Antiviral Activities of Syndecan- and Lectin-Agonists and AntagonistsPreclinical Development of Anti-HIV Microbicidal PeptidesIn collaboration with the Chisari laboratory, we recently identified a short peptide called C5A, which represents a novel class of HIV microbicidal candidates. C5A neutralizes HIV at an nM-µM range without apparent cytotoxicity to human cells. C5A corresponds to a small (18 amino acids) N-terminal region (amino acids 3-20) of the hepatitis C virus (HCV) nonstructural protein 5A (NS5A) (Figure 3). The sequence of C5A encompasses the region responsible for the anchoring of NS5A into the ER membrane. Importantly, in contrast to C5A (18 amino acids), full length NS5A (477 amino acids) does not inhibit HIV infection. We demonstrated that C5A disrupts the HIV membrane, but preserves the integrity of the cellular plasma membrane. The HIV membrane rupture is specific because C5A does not disturb the integrity of the plasma membrane of human cells even when used at high doses and because it does not inhibit the infection of other enveloped viruses such as influenza and vesicular stomatitis viruses. C5A possesses multiple attractive microbicidal properties: it i) blocks HIV infection of primary targets including T cells, macrophages and dendritic cells; ii) exhibits a broad range of antiviral activity against primary HIV isolates, multi-drug resistant HIV isolates; iii) interrupts an ongoing T cell infection; iv) prevents transmigration of HIV through primary human genital epithelial cells; v) blocks infection of dendritic and Langerhans cells ex vivo (skin tissues); vi) prevents HIV transfer from dendritic and Langerhans cells to T cells ex vivo; vii) is extremely efficacious since less than 15 min of exposure suffices for C5A to neutralize HIV; viii) is potent for a considerable length of time both prior to (at least 1 h) and after (at least 1 h) addition of HIV to cells; ix) is potent at a low pH; x) is soluble in water at inhibitory concentrations; xi) is not toxic to commensal Lactobacilli present in the vaginal tract; xii) exhibits minimal adverse changes, inflammation and toxicity in cervicovaginal tissue in vivo; xiii) is not immunogenic; xiv) does not affect cellular signaling pathways; xv) apparently does not allow viral development resistance; xvi) efficiently blocks HIV infectivity when diluted in genital fluids; and most importantly xvii) vaginal application of C5A offers complete protection against a vaginal viral challenge in a humanized mouse HIV transmission model. Indeed, in collaboration with the Garcia laboratory, we showed that a single topical vaginal application of C5A offered complete protection in humanized mice. Thus, C5A represents the prototype of a new generation of microbicidal agents that may have promise for HIV prevention. Our laboratory is currently conducting a series of experiments aimed at identifying the component of the viral membrane to which C5A binds because the C5A ligand, which resides in the membrane of HIV, represents a potential target for the development of a novel class of anti-HIV therapies with an unusual mechanism of antiviral action. We also are optimizing the in vitro potency and in vivo safety of C5A by creating a second generation of peptides using the parental C5A peptide as the archetype. The most potent C5A derivates are currently assessed for safety and efficacy in the HIV vaginal transmission macaque model. Protective results would provide an additional proof-of-concept of the usefulness of topically applied microbicides, such as the microbicidal peptide candidate C5A, to prevent genital HIV transmission. 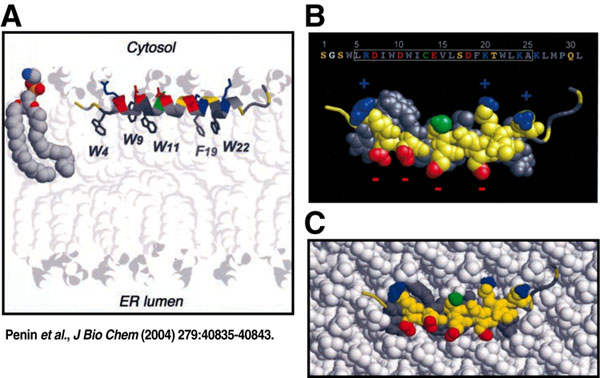 Figure 3: Structure of the HIV Microbicidal Candidate C5A. A. Positioning of the aa 5–25 amphipathic alpha-helix of NS5A (ribbon diagram) at the interface between polar heads and hydrophobic tails of phospholipids. As the average structure of NS5A (aa 1 to 31) was represented here, the position of side chain residues is only indicative. The phospholipid bilayer was drawn using the phosphatidyl-ethanolamine models. A phosphatidyl-ethanolamine molecule colored according to atom types (N, blue; O, red; P, yellow; C, H, gray) is given on the right to illustrate the polar head/hydrophobic tail interface. B. C5A corresponds to a short (18 amino acids) segment of HCV NS5A (477 amino acids) encompassing amino acids 3 to 20. Represented here is represented the amino acid sequence 1 to 31 of NS5A including the helix 5–25 (white box) and the space-filling representation of the polar and hydrophobic sides of the helix. Residues are color-coded according to their physico-chemical properties. Hydrophobic and polar residues are gray and yellow, respectively. Positive and negative charged groups of basic (Lys, Arg) and acidic (Asp, Glu) residues are blue and red, respectively. The SH group of Cys is green. C. Top view of C5A embedded at the interface of a model phospholipid membrane. Figure 3: Structure of the HIV Microbicidal Candidate C5A. A. Positioning of the aa 5–25 amphipathic alpha-helix of NS5A (ribbon diagram) at the interface between polar heads and hydrophobic tails of phospholipids. As the average structure of NS5A (aa 1 to 31) was represented here, the position of side chain residues is only indicative. The phospholipid bilayer was drawn using the phosphatidyl-ethanolamine models. A phosphatidyl-ethanolamine molecule colored according to atom types (N, blue; O, red; P, yellow; C, H, gray) is given on the right to illustrate the polar head/hydrophobic tail interface. B. C5A corresponds to a short (18 amino acids) segment of HCV NS5A (477 amino acids) encompassing amino acids 3 to 20. Represented here is represented the amino acid sequence 1 to 31 of NS5A including the helix 5–25 (white box) and the space-filling representation of the polar and hydrophobic sides of the helix. Residues are color-coded according to their physico-chemical properties. Hydrophobic and polar residues are gray and yellow, respectively. Positive and negative charged groups of basic (Lys, Arg) and acidic (Asp, Glu) residues are blue and red, respectively. The SH group of Cys is green. C. Top view of C5A embedded at the interface of a model phospholipid membrane.Development of a Novel Drug Delivery System for HIV Microbicides Although several anti-HIV agents showed promising microbicidal properties both in vitro and in vivo such as C5A, they have many obstacles to overcome. They must be stabilized against chemical degradation in a continuously warm and moist environment. C5A must also be delivered in a controlled manner to achieve the long-term and coitally independent efficacy necessary for prophylaxis in real world conditions. In collaboration with the Maskiewicz laboratory, we recently examined the anti-HIV activities of C5A after its formulation in a novel delivery system: subliming solid matrices. Subliming solids matrices are both chemically inactive and continuously hydrophobic, allowing labile peptides/proteins stored within, and releasing from, such matrices to be more stable than when stored in the solid state in moist air (Figure 4). A subliming solids-based delivery system can provide a broad range of release rates and durations independent of the presence of drug, and independent of the environment in which the device sublimes. Subliming matrices release incorporated peptides/proteins at the rate at which they sublime. We recently formulated C5A in cyclododecane solid matrices and tested them for cellular cytotoxicity and sustained release of C5A with preserved anti-HIV activities. 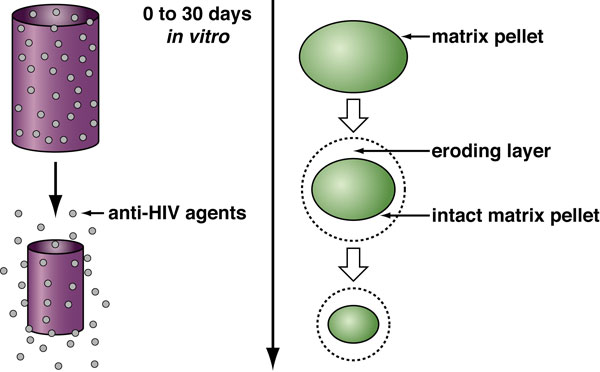 Figure 4. Model for Sublimation of Solid Matrices.We found that subliming solids do not exert any cellular toxicity to human cells. Importantly, C5A is efficiently released from the subliming matrices and remains constantly released over a period of 30 days in culture. More importantly, amounts of C5A released from subliming solids neutralize HIV over a period of 30 days. Subliming solids loaded with C5A were kept at room temperature for a month before use. This is an important feature for a microbicide considering that many developing countries, where microbicides are urgently needed, are poorly equipped in refrigerated storages. A same amount of non-formulated C5A placed under similar culture conditions lost its antiviral activity in less than a week. This suggests that formulation of C5A into subliming solids preserves its antiviral properties. By showing that subliming solids release C5A with potent anti-HIV activities for a sustained period of time, these promising preliminary data serve as a proof-of-concept that the unique attributes of biologically inert, subliming solids-based drug release could offer a significant opportunity to overcome a multitude of unresolved formulation problems intrinsic to a large panel of anti-HIV microbicides including C5A. Figure 4. Model for Sublimation of Solid Matrices.We found that subliming solids do not exert any cellular toxicity to human cells. Importantly, C5A is efficiently released from the subliming matrices and remains constantly released over a period of 30 days in culture. More importantly, amounts of C5A released from subliming solids neutralize HIV over a period of 30 days. Subliming solids loaded with C5A were kept at room temperature for a month before use. This is an important feature for a microbicide considering that many developing countries, where microbicides are urgently needed, are poorly equipped in refrigerated storages. A same amount of non-formulated C5A placed under similar culture conditions lost its antiviral activity in less than a week. This suggests that formulation of C5A into subliming solids preserves its antiviral properties. By showing that subliming solids release C5A with potent anti-HIV activities for a sustained period of time, these promising preliminary data serve as a proof-of-concept that the unique attributes of biologically inert, subliming solids-based drug release could offer a significant opportunity to overcome a multitude of unresolved formulation problems intrinsic to a large panel of anti-HIV microbicides including C5A.Ultimately, matrices will be incorporated into vaginal rings administrable to macaques, and evaluated for sustained C5A release, and most importantly for prolonged prevention of HIV transmission. Vaginal rings have been employed clinically for long-term delivery of contraceptives, and now this strategy is being applied for delivering subliming-solids-based formulations of C5A to protect against HIV. Unlike gels that must be used every day or at the time of sex, rings can be inserted into the vagina and provide retention and therapeutic activity for up to several months. Selected subliming matrices will be embedded into rings containing prefabricated “wells” to allow sustained C5A release and prolonged delivery system retention. Although the focus of our immediate effort is on the microbicidal candidate C5A, this novel delivery system is based on a release mechanism that is independent of the microbicide physiochemical properties. The platform is therefore easily adaptable to other drugs and drug combinations, allowing rapid development of devices that utilize the latest advances in microbicide-based HIV prophylaxis. |

