On Press:
AP-2 Not So Essential After All
By Jason Socrates
Bardi
Sometimes an accessory you thought was absolutely necessary
turns out not to be necessary after all.
Imagine that you thought that city buses were the only way
to get across town. The one thing you think you need is your
bus pass. Without your pass, how could you ride? But then
you lose your bus pass. You discover you can pay with loose
change. Or you can ride the subway. Or take taxis; pedicabs;
foot paths; even trolley cars. It turns out that the bus pass
is not so essential.
The findings published by two scientists at The Scripps
Research Institute (TSRI) this month in the Journal of
Cell Biology are somewhat analogous. In the report, Sean
Conner, a research associate, and Sandra Schmid, professor
and chair of the Department of Cell Biology, demonstrate that
clathrin-mediated endocytosis can still take place even when
a protein that was thought absolutely essential for the process
is lost.
Clathrin-mediated endocytosis is a cellular process whereby
important hormones, proteins, nutrients, and other macromolecular
"cargo" needed by a cell are collected into small transport
vehicles called 'coated vesicles' and carried into the cell.
Coated vesicles are derived from patches of membrane where
receptors are located and where the cargo molecules are gathered.
That patch of membrane becomes involuted, bulging inward to
form a pit that is surrounded on the inside of the cell by
a lattice-like coat of protein known as clathrin. The involuted
membrane pinches off, forming a clathrin-coated vesicle filled
with cargo.
Adaptor protein complex molecules (AP-2) are key components
in clathrin-mediated endocytosis because they trigger clathrin
assembly, interact directly with cargo molecules, and recruit
a number of other accessory factors. AP-2 molecules were believed
essential for clathrin-mediated endocytosis.
In their paper, however, Conner and Schmid disrupted the
function of AP-2 molecules by overexpressing an enzyme called
the adaptor-associated kinase (AAK1), which phosphorylates
AP-2 and modulates its function. Too much AAK1 means not enough
AP-2, and they assumed that limiting AP-2 would negatively
affect clathrin-mediated endocytosis. To their surprise, while
some cargo molecules were no longer taken up by the cell,
the clathrin-mediated uptake of other cargo molecules was
unaffected.
This work suggests the AP-2 molecule may not be stoichiometrically
required for coat assembly, as previously thought, and that
AP-2 molecules may be just one class of adaptor proteins that
function to couple clathrin coat assembly with the efficient
packaging of cargo molecules.
To read the article, "Differential requirements for AP-2
in clathrin-mediated endocytosis" by Sean D. Conner and Sandra
L. Schmid, please see the Journal of Cell Biology,
162, 773-780 or go to http://www.jcb.org/cgi/content/abstract/162/5/773.

|
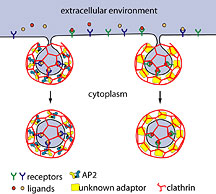
Adaptor proteins drive
the assembly of clathrin coats at the plasma membrane and
coordinate the selective packaging of receptor/ligand complexes
into clathrin-coated vesicles for internalization. Click
to enlarge.
|

