The Body Clock Revisited
By Jason Socrates
Bardi
In Shakespeare's Hamlet, the troubled young prince
says longingly of sleep, "Tis a consummation devoutly to be
wished."
Sleep, for Hamlet, signifies more than just sleep, but his
lamentation still rings true to anyone who has suffered from
lack of sleep. People who are jet lagged after a long plane
flight or who are feeling wiped out after working a night
shift know what it feels like to wish devoutly for sleep.
Their bodies cry: Something is rotten in Denmark.
Shift workers and world travelers suffer because the body
follows a daily cycle known as a circadian rhythm. Humans,
mice, and other organisms have internal body "clocks" to keep
track of time and coordinate biological processes to the rhythm
of day and night. For instance, our blood pressure fluctuates
daily, rising and falling at predictable times of the day
or night.
Our bodies accomplish this through receptors that detect
light, but scientists did not know which receptors were responsible
for this until recently. Some scientists had speculated that
the key receptor would be rhodopsin, the light-capturing protein
present in rods and cones of the eye that are responsible
for vision. However, certain people who were born blind because
they lacked rods and cones could still maintain their circadian
balance.
In recent months, scientists from The Scripps Research Institute
(TSRI) and the Genomics Institute of the Novartis Research
Foundation (GNF) have demonstrated that a gene called Opn4,
which codes for a protein receptor called melanopsin, is responsible
for keeping circadian rhythms entrained to a 24-hour day—like
a tiny key that winds a grandfather clock.
A study by the TSRI and GNF team that appeared in Science
last December demonstrated melanopsin is responsible for keeping
the body in sync with the day. In the Science study the TSRI
and GNF team knocked out the Opn4 gene in mice and
showed that in its absence, the mice could not reset their
clocks.
Now another Science article by the same team tests
the role of melanopsin in visually blind mice. The TSRI and
GNF team created a model with no visual photoreceptors and
no melanopsin. Then they looked at how the sleep–wake
cycle was affected. Not surprisingly, they found that these
models cannot adjust their body clocks to the day–night
cycle—their body clocks run like a runaway train. However,
the controls who were visually blind but did have melanopsin
had no such problems. This research demonstrates how critical
melanopsin is for maintaining circadian rhythms, and it may
help in the development of strategies for correcting sleep
disorders, many of which are related to circadian rhythms.
Furthermore, understanding the protein that resets the body's
clock should help in research aimed at countering the most
common circadian problems including jet-lag and night shifts.
The article, "Melanopsin is Required for Non-Image Forming
Responses in Blind Mice," will be published by the journal
Science and will appear on its rapid electronic
publication web site on June 26, 2003. The article will
appear in print later this year.

|
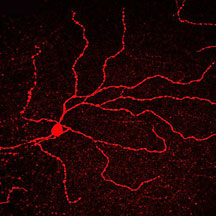
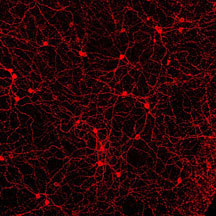
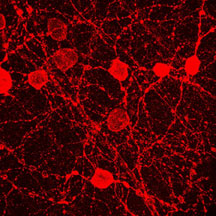
Micrographs of melanopsin in neuronal
cells. Top panel shows a single fluorescently-labeled melanopsin-producing
neuron. The middle panel shows a network of such neurons,
and the bottom panel shows a close-up of this network. Images
courtesy Satchidananda Panda, GNF.
|

