Attachment Receptors and Hot Spots for HIV Infection
By Jason Socrates
Bardi
In the world of human immunodeficiency virus (HIV) research,
much of the focus for the last couple of decades has been
on the host receptors that are necessary for the entry of
HIV into the cell—the CD4 receptor and its co-receptor
CCR5, for instance, which HIV uses to enter helper T cells.
So much attention has been given to these CD4 receptors,
in fact, that they have become almost synonymous with HIV
itself. Loss of CD4 receptors has long been a defining diagnostic
of AIDS, and the level of CD4 cells is a marker of disease
progression.
However, this is only the start of the story. There are
also "attachment" receptors, which have been shown in recent
years to enhance the entry of HIV into cells.
"Now more than ever, there is a growing body of knowledge
that suggests attachment receptors can have a profound impact
on HIV pathogenesis," says Assistant Professor Philippe Gallay,
who is a member of the Department of Immunology at The Scripps
Research Institute (TSRI). In his laboratory, Gallay looks
at the attachment of the virus to cells and looks toward using
those host proteins as a guide for drug design.
Hooked on Sugars
The particular class of attachment receptor that interests
Gallay consists of long, extended proteins on the surface
of human cells that are decorated with a kind of sugar known
as heparin sulfate chains. Heparin sulfate chains are attached
to "glyco" proteins on the surface of cells like macrophages,
which use them for a number of biological reasons, like binding
to cytokines and growth factors in the bloodstream.
Gallay and his colleagues showed a few years ago that these
heparin sulfate chains are also important players in the pathology
of HIV because cells that are decorated with these heparin
sulfate chains are like glue for the virus. Heparin sulfate
chains have affinity for the viral coat protein GP120 on HIV,
and HIV seems to use them to gain entry into cells like macrophages,
one of the virus's main target cells.
"When you remove these sugars, the virus cannot infect macrophages,"
says Gallay, who demonstrated this a few years ago in a study
with Research Associate Andrew C. S. Saphire.
But the sugars are only part of the story. Recently, Gallay
and members of his laboratory published a paper describing
the primary importance of a human protein called syndecan,
which contains certain "motifs" of amino acids that the body's
heparin sulfate chains attach to, which in turn interact with
HIV's viral coat protein GP120. The group that published this
finding included Michael D. Bobardt, Saphire, Hsiu-Cheng Hung,
and Xiaocong Yu at TSRI and their colleagues Bernadette Van
der Schueren, Zhe Zhang, and Guido David of the Center for
Human Genetics at the University of Leuven and Flanders Interuniversity
Institute in Belgium.
HIV CoOpts the Machinery of the Cell
Syndecans are actually a family of four different highly
conserved transmembrane proteins that sit on the outside of
cells. While no syndecan structures have been solved yet,
their extracellular domains are known to extend from the cell
surface, and at their terminus, they usually have motifs to
which heparin sulfate chains are covalently attached.
Syndecans are the connection between the cells and the extracellular
matrix, the molecular scaffold the body uses to build collections
of cells into tissues. The interaction of syndecans with extracellular
matrix components induces signals inside cells that are related
to adhesion and migration. Cells that are adhering to the
extracellular matrix express high levels of syndecans, while
T cells and other mobile cells that do not adhere to the extracellular
matrix do not express syndecans.
"They [Syndecans] probably have several biological roles,"
says Gallay, "such as presenting cytokines and growth factors
to receptors."
Significantly, this machinery that binds to cytokines and
growth factors in the bloodstream is what HIV has hijacked
for its own purposes. It tricks the syndecans into capturing
virions rather than growth factors.
"We found that syndecans can capture a lot of virus via
their long extended chains," says Gallay. He had previously
shown that syndecans are expressed on the surface of macrophages
and that, by removing the syndecans, they were able protect
the macrophages from infection. He also showed that monocytes,
the precursor cells from which macrophages are derived, do
not express syndecans and cannot be infected with HIV, whereas
macrophages do express syndecans and can be infected. So syndecans,
because of the affinity of their heparin sulfate chains for
GP120, can be used by HIV to gain entry into cells.
But there is more. In his recent study, published in the
January 2003 issue of the journal Immunity, Gallay and his
colleagues showed that syndecans could act as in trans receptors,
meaning that cells decorated with syndecans not only capture
HIV, they help it to infect other cells that do not express
the syndecans.
In other words, the presence of syndecans on one cell can
help HIV enter another cell.
1 | 2 |

|
Assistant Professor Philippe Gallay
(left) and Research Associate Andrew C. S. Saphire examine
a gel. Photo by Michael Balderas.
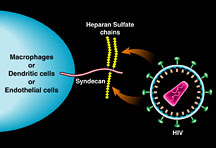
This drawing illustrates
the interaction between HIV and heparin sulfate chains on
the surface of human cells. Courtesy Peggy Myer, Biomedical
Graphics.Click
to enlarge.
|

