Rac Activation and Cell Motility
By Jason Socrates
Bardi
Anyone who has ever seen a video of a neutrophil chasing
a bacterium cannot help but to be impressed with the persistence
of these phagocytic blood cells. Like a cat chasing a mouse,
the neutrophil chases the bacterium around the "corners" of
cells and other obstacles until it catches, engulfs, and destroys
the pathogen as part of the body's innate immune response.
Behind this amazing microscopic drama is the important physiological
phenomenon of cell motility. In addition to its crucial role
in the innate immune response, cell motility is important
for such diverse physiological situations as wound healing,
angiogenesis, metastasis in cancer, and neuronal development.
Scientists have for years sought the master regulators of
cell motility—the molecules driving the process that
have their hands on the steering wheels and feet on the gas
pedals.
When cells move, their movement is driven by the assembly
and polymerization of actin at the leading edge of the cells
and the myosin-mediated contraction in the tail of the cells.
The dynamics of both of these processes must be highly orchestrated
so that the cell can move smoothly, change directions, and
stop.
In a recent paper published in the journal Current Biology,
Professor Gary Bokoch and his colleagues at The Scripps Research
Institute show that one of the molecules that is controlling
these dynamics may be Rac, a small GTP-binding protein. Bokoch
and his colleagues found that Rac is spatially and temporally
regulated to coordinate leading-edge extension and tail contraction
during the "chemotactic" motility of human neutrophils.
Using a fluorescence resonance energy transfer-based technique,
Bokoch and his colleagues were able to detect the formation
of active Rac-GTP and show that Rac is dynamically activated
during motility. Specifically, Bokoch and his colleagues showed
that Rac is activated at specific times and in specific locations
in the extending leading edge. In conjunction with data obtained
by introduction of mutant Rac proteins, they propose that
Rac establishes and maintains the leading edge of crawling
neutrophils.
Surprisingly, the group also found activated Rac in the
retracting tail of motile neutrophils, suggesting that Rac
might be involved in the contraction events that pull the
retracting tail forward. This was verified by demonstrating
that an inhibitory form of Rac blocked tail retraction. Bokoch
and his colleagues also found that Rac activity is modulated
by cell adhesion, suggesting that integrin-mediated signals
probably play important roles in regulating Rac activation
during motility.
To read the article, "Spatial and Temporal Analysis of Rac
Activation during Live Neutrophil Chemotaxis" by Elisabeth
M. Gardiner, Kersi N. Pestonjamasp, Benjamin P. Bohl, Chester
Chamberlain, Klaus M. Hahn, and Gary M. Bokoch, please see:
http://www.current-biology.com/cgi/content/abstract/12/23/2029/

|
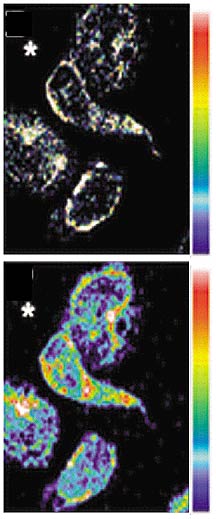
These confocal images of human neutrophils
stained for F-actin (top) and Rac2 antibody (bottom) show
that Rac2 becomes activated and re-localizes to areas of actin
polymerization. The relative intensity is shown on the attached
color scale from blue (low) to red (high).
|

