How Do You Solve Relief—Structure of Pain-Modulating
Enzyme Described by TSRI Scientists
A group of researchers from The Scripps Research Institute
(TSRI) has solved the structure of an enzyme that modulates
central nervous system (CNS) functions such as pain perception,
cognition, feeding, sleep, and locomotor activity.
The enzyme, described in the November 29th issue of the
journal Science, is called fatty acid amide hydrolase
(FAAH), and it breaks down certain fatty signaling molecules
that reside in the lipid membranes of CNS cells. The TSRI
group reports that FAAH modulates the action of these fatty
signaling molecules through an unusual mechanism of action
whereby it scoops them out of the cell membranes and chews
them up.
"I envision that if someone could make a specific inhibitor
to FAAH, you could, in principal, get pain relief without
any of the side effects," says Associate Professor Benjamin
Cravatt, one of the paper's lead authors and an investigator
in TSRI's Department of Cell Biology, Department of Chemistry,
and The Skaggs Institute for Chemical Biology.
"As soon as we had the view of the active site, we knew
FAAH could be used to make lead clinical candidates," adds
Raymond Stevens, who is a professor in the Department of Molecular
Biology and Department of Chemistry at TSRI and the other
lead author on the paper. "The deep pocket with well-defined
cavities provides the guidance to take the currently available
tight binding inhibitors and improve on their specificity
and pharmakokinetic properties."
Pain Management and FAAH
Easing pain is practically synonymous with practicing medicine,
and since before the days of Hippocrates, doctors have sought
the best ways of doing this, looking for compounds that not
only ease pain, but do so as fast, effectively, and lastingly
as possible—without unwanted side effects.
Every analgesic, from opiates to hypnotism to electroshocks
to balms, has side effects, and therein lies the rub, whether
relieving the pain or the side effects is more pressing.
One compound that has been hotly debated in the last 10
years is delta-9-tetrahydrocannabinol (THC), the active ingredient
in marijuana. The reason THC works is that it mimics the action
of natural cannabinoids that the body produces in signaling
cascades in response to a peripheral pain stimulus. THC binds
to "CB-1" receptors on cells on the rostral ventromedial medulla,
a pain-modulating center of the brain, decreasing sensitivity
to pain.
Unfortunately, the receptors that THC bind to are also widely
expressed in other parts of the brain, such as in the memory
and information-processing centers of the hippocampus. Binding
to nerve cells of the hippocampus and other cells elsewhere
in the body, THC creates a range of side effects as it activates
CB-1 mediated signaling—including distorted perception,
difficulty in problem-solving, loss of coordination, increased
heart rate and blood pressure, anxiety, and panic attacks.
The challenge posed by THC and other cannabinoids is to
find a way to use them to produce effective, long-lasting
relief from pain without the deleterious side effects. Now
Cravatt and Stevens think they know just how to do that.
The solution, as they see it, is to increase the efficacy
of the natural, endogenous cannabinoids ("endocannabinoids")
the body produces to modulate pain sensations.
"When you feel pain, you release endocannabinoids [which
provide some natural pain relief]," says Cravatt. "Then the
amplitude and duration of their activity are regulated by
how fast they are broken down."
In particular, the body releases an endogenous cannabinoid
called anandamide, a name derived from the Sanskrit word meaning
"internal bliss." When the body senses pain, anandamide binds
to CB-1 and nullifies pain by blocking the signaling. However,
this effect is weak and short-lived as FAAH quickly metabolizes
the anandamide—the compound has a half-life of only a
few minutes in vivo.
In some ways, THC is superior to anandamide as a pain reliever
because it is not as readily metabolized by FAAH. But THC
goes on to suppress cannabinoid receptor activity all over
the body. This, coupled with the fact that it is a controlled
substance, makes THC an unattractive target for developing
therapeutics.
FAAH is much more attractive target for pain therapy because
inhibiting FAAH would increase the longevity of anandamide
molecules—preventing their breakdown and allowing them
to continue providing some natural pain relief.
The structure that Cravatt, Stevens, and their TSRI colleagues
solved should form a template for designing specific inhibitors
that control the action of FAAH when the body is sensing pain
and releasing anandamide.
The research article, "Structural Adaptations in a Membrane
Enzyme that Terminates Endocannabinoid Signaling" is authored
by Michael H. Bracey, Michael A. Hanson, Kim R. Masuda, Raymond
C. Stevens, and Benjamin F. Cravatt, and appeared in the November
29, 2002 issue of the journal Science.
The research was funded by the National Institute on Drug
Abuse, the Searle Scholars Program, The Skaggs Institute for
Chemical Biology, a National Research Service Award, and a
Jabinson graduate fellowship.

|
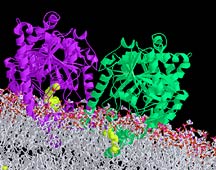
This ribbon diagram shows the enzyme
fatty acid amide hydrolase (FAAH), whose structure was recently
solved by TSRI scientists. FAAH is an attractive target for
developing drugs to provide pain relief. Click
to enlarge.
|

