Research Brief:
Three Seminal Reactions from the Sharpless lab
Asymmetric
Epoxidation (AE)
Reaction Scheme:
|
|
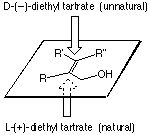
Facial Selectivity:
|
|
 |
 |
 |
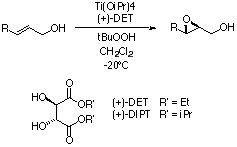
The Asymmetric Epoxidation, or AE, involves the conversion
of an allylic alcohol to an epoxy alcohol. Titanium (IV) isopropoxide
is used as a catalyst and (+) or (-) diethyl or diisopropyl
tartrate as a chiral ligand. Use of a chiral ligand allows
t-butyl hydroperoxide to deliver an oxygen stereospecifically
to the olefin, regardless of substitution pattern. Enantiomeric
excesses are generally above 90%, often above 98%. Yields
can range from 50% to 99%.

Kinetic Resolution:
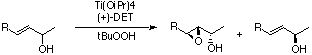
With a slight modification of the procedure, it is possible
to effect a kinetic resolution of racemic allylic alcohols.


References:
For the original reference see:
 |
Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980,
102, 5974. |
For the paper describing kinetic resolution see:
 |
Martin, V. S.; Woodward, S. S.; Katsuki, T.; Yamada,
Y.; Ikeda, M.; Sharpless, K. B. J. Am. Chem. Soc. 1981,
103, 6237. |
For good reviews see:
 |
Finn, M. G.; Sharpless, K. B. Asymm. Synth. 1985,
5, 247. |
 |
Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune,
H.; Sharpless, K. B. J. Am. Chem. Soc. 1987,
109, 5765. |
Top
Asymmetric
Dihydroxylation (AD)
Reaction Scheme:
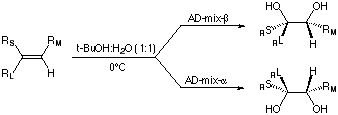
The Asymmetric Dihydroxylation involves the conversion of
a substituted alkene to a diol. Osmium tetroxide is used as
a catalyst and one of the various cinchona ligands is used
to enantioselectively deliver the the oxygens to the olefin.
Facial Selectivity:
The facial selectivity is easy to predict using a simple mnemonic
shown graphically below.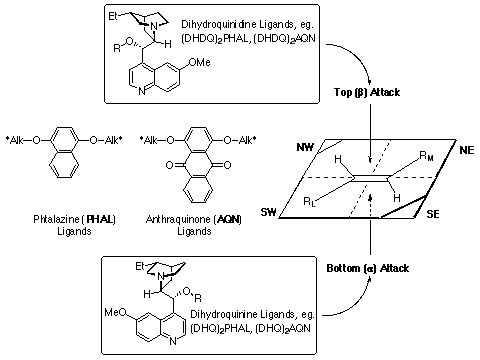 

References:
For the original reference on catalytic AD:
 |
Jacobsen, E. N.; Marko, I.; Mungall, W. S.; Schroder,
G.; Sharpless, K. B. J. Am. Chem. Soc. 1988, 110,
1968. |
For the original reference on using a two phase system and
K3Fe(CN)6 as the oxidant:
 |
Kwong, H. L.; Sorato, C.; Ogino, Y.; Chen, H.; Sharpless,
K. B. Tetrahedron Lett.1990, 31,
2999. |
For the authoritative review see:
 |
Kolb, H.; VanNiewenhze, M. S.; Sharpless, K. B. Chem.
Rev.1994, 94, 2483-2547. |
Top
Asymmetric
Aminohydroxylation (AA)
Reaction Scheme:
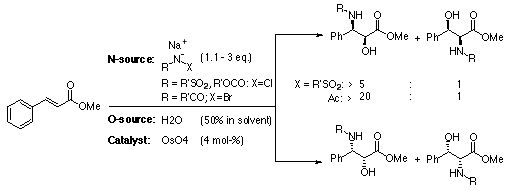
The Asymmetric Aminohydroxylation involves the conversion
of a properly substituted alkene to an amino alcohol. Osmium
tetroxide is used as a catalyst and one of the various cinchona
ligands is used to enantioselectively deliver the the heteroatoms
to the olefin. The cinchona ligands are responsible for not
only enantioselectivity, they also improve regio- and chemoselectivity.
Water is used as an oxygen source, and there are several possible
nitrogen sources.
Selectivity:
The ratio of of the two constitutional isomers is dependant
largely on the substrate, but the regioselectivity can be controlled
to a degree by using the appropriate solvent and ligand. (vide
infra) Facial selectivity can be determined using the simple
mnemonic adopted from the AD reaction.
 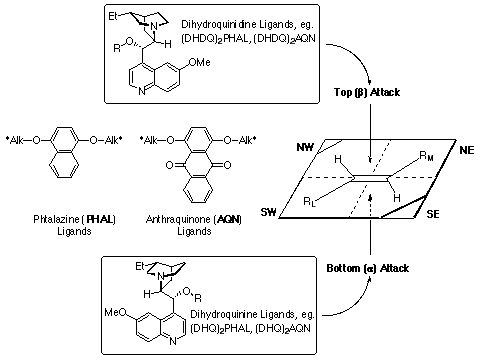 
References:
For the original reference see:
 |
Li, G.; Chang, H.-T.; Sharpless, K. B. Angew. Chem.
Int. Ed. Engl.1996, 35, 451. |
Smaller Nitrogen Sources Are Better:
 |
Rudolph, J.; Sennhenn, P. C.; Vlarr, C. P.; Sharpless,
K. B. Angew. Chem. Int. Ed. Engl.1996, 35,
2810. |
N-Halocarbamate Salts:
 |
Li, G.; Angert, H. H.; Sharpless, K. B. Angew. Chem.
Int. Ed. Engl.1996, 35, 2813. |
For good reviews see the following books:
 |
H. Becker and K. B. Sharpless, Asymmetric Dihydroxylation,
in "Asymmetric Oxidation Reactions: A Practical Approach
in Chemistry", ed. by T. Katsuki, Oxford University
Press 2001. |
 |
H. C. Kolb and K. B. Sharpless, Transition Metals
for Fine Chemicals and Organic Synthesis ed. by M.
Beller and C. Bolm, Wiley-VCH 1998. |
Top

|
|

