|
(page 2 of 2)
The Assay
Quigley uses an assay that has the advantages of being inexpensive,
fast, simple, not requiring complicated surgeries, animal
protocols, and large spaces.
The assay itself uses chicken eggs with their shells removed
that are placed in an incubator to develop for a few days.
Because the eggs are only a few days old, they are immunologically
naïve and will tolerate human tumor cells.
After ten days, a tumor is placed on the soft chorioallantoic
membrane on the inside of the shell and an antibody against
the tumor cells is injected into the egg. Quigley uses an
aggressive tumor cell that will grow and form metastases in
under a week. An antibody of interest can be injected into
the egg and tested for the ability to block metastasis. Another
advantage of this model is that the volume is small and dilution
of antibody over the course of the assay is minimal. "It stays
in and does its job," says Quigley.
After a week with the tumor, metastatic cells can be detected
by looking for evidence of human (tumor) DNA in a part of
the egg that is distinct from where the tumor was implanted
a week before. Human DNA has thousands of copies of 30 to
50 base "alu" repeats spread throughout its genome, and finding
copies of these alu repeats is analogous to finding a visible
tumor. The DNA can be extracted from the sample using a simple
prep and amplified through the polymerase chain reaction with
alu-specific primers.
Then the amount of DNA extracted from the sample can be
quantified by comparing the signal to a standard curve. The
number of copies of alu repeats progresses linearly with the
number of tumor cells. "We can detect anywhere from 100 to
10,000 cells per sample," says Quigley. "And 100 cells is
really seeing micrometastasis—cells that you might never
see [looking at sections under a microscope]."
By testing different antibodies against a control, those
which alter the metastatic phenotype of the tumor cells can
be easily identified. Once an antibody is found that modulates
metastasis, its antigen must then be identified by using the
same antibody to isolate the correct protein, which then gets
sequenced.
Occasionally a protein is found that, when blocked, stops
metastasis. Quigley's group has found several of these thus
far. Some were to be expected, such as a membrane-spanning
integrin-associated protein they identified that is necessary
for helping the integrins loose their grip on other cells
and on the extracellular matrix, an important first step in
metastasis.
Others, however, have turned out to be a surprise. One antigen
that was identified was that of an novel protein whose cDNA
has been sequenced in an expression database but not yet annotated.
"We have no idea what it is," Quigley says. "But we have
cloned it, and we are trying to find out how it works."
Metastasis and Angiogenesis—The Complete Picture
In addition to metastasis, Quigley's laboratory studies
angiogenesis, the process where blood vessels are formed and
differentiated. The goal of both the metastasis and the angiogenesis
work is to identify molecules that could become targets for
intervention, and he employs the same basic techniques in
both, using subtractive immunization and chicken egg in
vivo models to study them.
In the angiogenic models, blood vessels can be easily counted
and observed under a microscope, and this forms a basic assay
that can then be used to screen for compounds that inhibit,
stimulate, or otherwise modulate the angiogenic process.
In fact, the laboratory's first major paper in the field,
recently published in Blood, was about growth factor
induced angiogenesis. After implanting native collagen onto
a chick embryo, they injected angiogenesis growth factors
into the collagen, and then studied the effect of new vessel
growth on the surrounding area to uncover the active molecules
that contributed to the remodeling of the new tissue. Some
of the active molecules turned out to be enzymes that break
down proteins, the same proteolytic enzymes that Quigley had
been studying for years as part of his research into the tumor
invasion process. Now his research has turned up the fact
that the same enzymes are also involved in the formation of
new blood vessels, a gratifying breakthrough.
In other instances, as well, these two problems merge. For
instance, tumor angiogenesis—the process whereby tumors
will cause a proliferation of blood vessel growth—is
an important first step in metastasis. A tumor placed on a
membrane will cause new vessels to grow into a drop of collagen
that is laid on top of the tumor, and this process can be
studied.
"In some cases," he notes, "all the areas in the laboratory
merge."
1 | 2 |

|
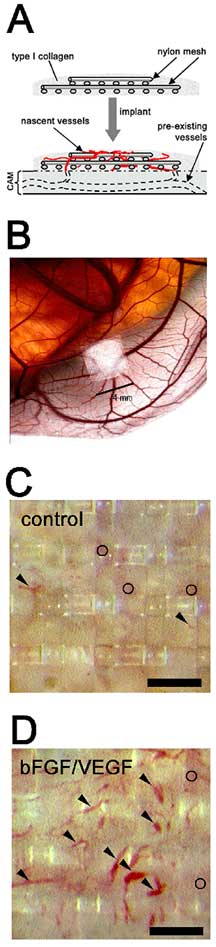
Basis for quantifying new blood vessel growth (angiogenesis)
involves implanting a gridded nylon mesh surrounded by collagen
onto the chorioallantoic membrane of a chick embryo (A and
B). When the collagen contain specific growth factors, bFGF
and VEGF, enhanced appearance of new blood vessels occur in
the upper grid of the nylon mesh (C vs. D), which can be easily
quantified.
|

