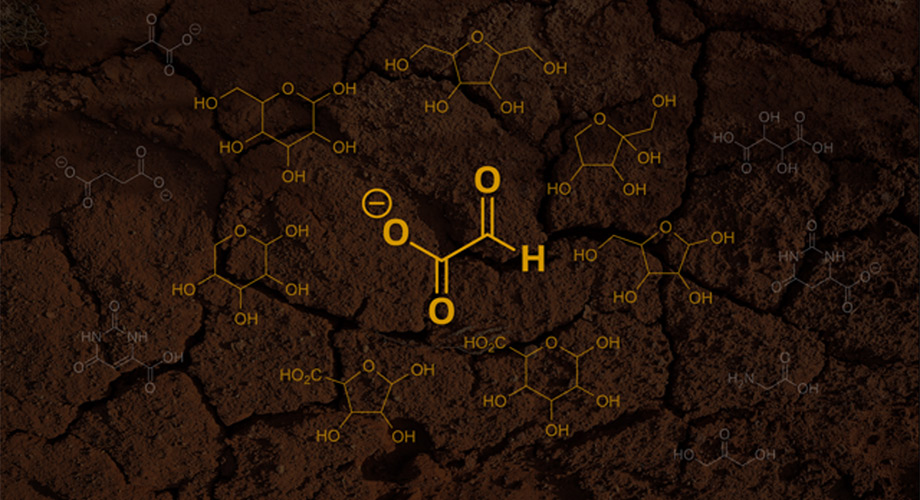
A new hypothesis states that the first sugars emerged from glyoxylate (pictured as the center molecule). In this hypothesis, glyoxylate first reacts with itself and then the byproducts from these reactions, ultimately forming simple sugars and other products (pictured as the surrounding molecules). Credit: Scripps Research and Unsplash
Where did the first sugars come from?
Origin-of-life chemists from Scripps Research and the Georgia Institute of Technology propose that glyoxylate could have been the original source of sugars on the “prebiotic” Earth.
April 13, 2023
LA JOLLA, CA— Two prominent origin-of-life chemists have published a new hypothesis for how the first sugars—which were necessary for life to evolve—arose on the early Earth.
In a paper that appeared on April 13, 2023, in the journal Chem, the chemists from Scripps Research and the Georgia Institute of Technology propose that key sugars needed for making early life forms could have emerged from reactions involving glyoxylate (C2HO3–), a relatively simple chemical that plausibly existed on the Earth before life evolved.
“We show that our new hypothesis has key advantages over the more traditional view that early sugars arose from the chemical formaldehyde,” says Ramanarayanan Krishnamurthy, PhD, a professor in the Department of Chemistry at Scripps Research.
Krishnamurthy’s co-author was Charles Liotta, PhD, Regents' Professor Emeritus at the Georgia Institute of Technology’s School of Chemistry and Biochemistry.
Origin-of-life chemists seek to explain how the basic molecular building blocks and reactions necessary for life could have arisen from the simple chemicals that were likely present on the “prebiotic” Earth. The overarching aim of the field is to answer the fundamental question of how our living planet came to be. But its discoveries also can inform—and have informed—many other fields, from atmospheric science and geology to synthetic biology and the search for life on other planets.
The three major classes of biological molecule whose availability needs to be explained by origin-of-life chemistry are: the amino acids that make up proteins, the nucleobases that make up the “letters” of DNA and RNA, and the sugars (also called carbohydrates) that are found throughout biology, including in the twisted backbone structure of DNA and RNA. According to the prevailing theories, amino acids probably arose from ammonia (NH3), while nucleobases arose from hydrogen cyanide (HCN).
The origin of sugars has been less clear. Many scientists believe the first sugars came from reactions involving formaldehyde (CH2O), but this theory has some drawbacks.
“The formaldehyde reactions proposed by this theory are quite messy—they have uncontrolled side reactions and other drawbacks due to formaldehyde’s high reactivity under the envisioned early-Earth conditions,” Liotta says.
The chemists’ proposed alternative is a “glyoxylose reaction” scenario in which glyoxylate first reacts with itself, forming a close cousin of formaldehyde known as glycolaldehyde. The researchers suggest that glyoxylate, glycolaldehyde, their byproducts and other simple compounds could have continued to react with one another, ultimately yielding simple sugars and other products—without the drawbacks of formaldehyde-based reactions.
Glyoxylate already has a prominent role in origin-of-life chemistry theories. Swiss chemist Albert Eschenmoser proposed in 2007 that a form of it might have been the source for multiple original biomolecules. Krishnamurthy and Furman University chemist Greg Springsteen, PhD, also suggested in a 2020 Nature Chemistry paper that glyoxylate could have helped initiate a primordial version of the modern (tricarboxylic acid) TCA cycle, a basic metabolic process found in most life forms on Earth.
Krishnamurthy and his team are currently working to demonstrate in the laboratory that the glyoxylose reaction scenario could indeed have yielded the first sugars.
“Such a demonstration would expand the role of glyoxylate as a versatile molecule in prebiotic chemistry and further stimulate the search for its own origin on the prebiotic Earth,” Krishnamurthy says.
The chemists are also looking into potential commercial applications of reactions that make glyoxylate, since these effectively consume CO2 and thus can be used to reduce CO2 levels, either locally in industrial settings or globally to combat global warming.
The study, “The Potential of Glyoxylate as a Prebiotic Source Molecule and a Reactant in Protometabolic Pathways – The Glyoxylose Reaction,” was supported by the NASA Exobiology Program NNH20ZA001N-EXO (20-EXO-0006) and by the National Science Foundation and the NASA Astrobiology Program under the Center for Chemical Evolution (CHE-1504217).
For more information, contact press@scripps.edu

