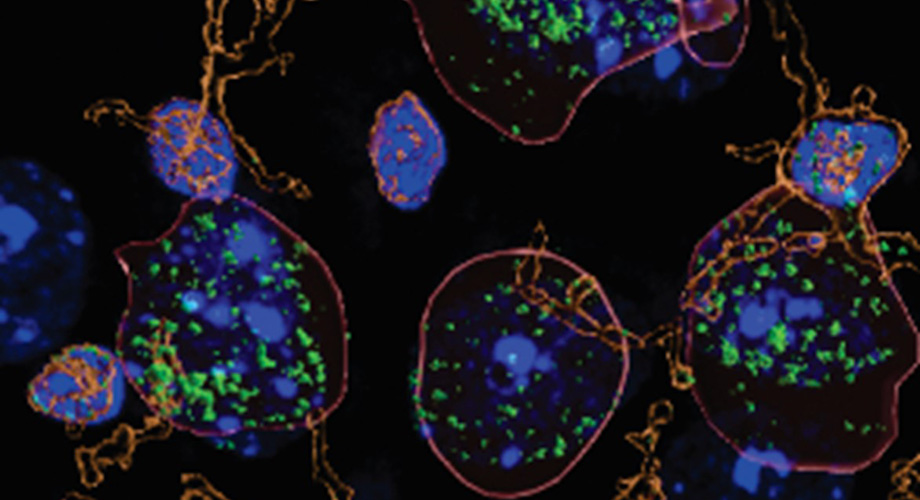
Researchers discovered higher than usual levels of the immune signaling molecule interleukin-1B (green) in neurons (outlined in red) from the brains of alcohol-dependent mice. Credit: Scripps Research
How heavy alcohol consumption increases brain inflammation
The findings by a Scripps Research team point toward a potential new drug target for treating alcohol use disorder.
March 06, 2023
LA JOLLA, CA—For people with alcohol use disorder (AUD), there is a constant, vicious cycle between changes to the brain and changes to behavior. AUD can alter signaling pathways in the brain; in turn, those changes can exacerbate drinking.
Now, scientists at Scripps Research have uncovered new details about the immune system’s role in this cycle. They reported in the journal Brain, Behavior and Immunity on Feb. 28, 2023, that the immune signaling molecule interleukin 1β (IL-1β) is present at higher levels in the brains of mice with alcohol dependence. In addition, the IL-1β pathway takes on a different role in these animals, causing inflammation in critical areas of the brain known to be involved in decision-making.
“These inflammatory changes to the brain could explain some of the risky decision-making and impulsivity we see in people with alcohol use disorder,” says senior author Marisa Roberto, PhD, the Schimmel Family Chair of Molecular Medicine and a professor of neuroscience at Scripps Research. “In addition, our findings are incredibly exciting because they suggest a potential way to treat alcohol use disorder with existing anti-inflammatory drugs targeting the IL-1β pathway.”
AUD is characterized by uncontrolled and compulsive drinking, and it encompasses a range of conditions including alcohol abuse, dependence and binge drinking. Researchers have previously discovered numerous links between the immune system and AUD—many of them centered around IL-1β. People with certain mutations in the gene that codes for the IL-1β molecule, for instance, are more prone to developing AUD. In addition, autopsies of people who had AUD have found higher levels of IL-1β in the brain.
“We suspected that IL-1β was playing a role in AUD, but the exact mechanisms in the brain have been unclear,” says first author Florence Varodayan, PhD, an assistant professor at Binghamton University and former postdoctoral fellow in the Roberto lab.
In the new study, Roberto, Varodayan and their colleagues compared alcohol-dependent mice with animals drinking moderate or no alcohol at all. They discovered that the alcohol-dependent group had about twice as much IL-1β in the medial prefrontal cortex (mPFC), a part of the brain that plays a role in regulating emotions and behaviors.
The team then went on to show that IL-1β signaling in the alcohol-dependent group was not only increased, but also fundamentally different. In mice that had not been exposed to alcohol, as well as in mice that had drunk moderate amounts of alcohol, IL-1β activated an anti-inflammatory signaling pathway. In turn, this lowered levels of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), a signaling molecule known to regulate neural activity in the brain.
However, in alcohol-dependent mice, IL-1β instead activated pro-inflammatory signaling and boosted levels of GABA, likely contributing to some of the changes in brain activity associated with AUD. Notably, these changes in IL-1β signaling in the alcohol-dependent mice persisted even during alcohol withdrawal.
Drugs that block the activity of IL-1β are already approved by the U.S. Food and Drug Administration to treat rheumatoid arthritis and other inflammatory conditions. More work is needed to determine whether these existing drugs could have utility in treating AUD.
“We plan to follow up on this study with more work on exactly how targeting specific components of the IL-1β pathway might be useful in treating alcohol use disorder,” says Roberto.
In addition to Roberto and Varodayan, authors of the study, “Chronic ethanol induces a pro-inflammatory switch in interleukin-1β regulation of GABAergic signaling in the medial prefrontal cortex of male mice,” include Pauravi Gandhi, Michal Bajo, Michael Steinman and Amanda Roberts of Scripps; Amanda Pahng and Scott Edwards of Louisiana State University; Tony Davis and Michael Burkart of University of California, San Diego; William Kiosses of La Jolla Institute for Immunology; and Yuri Blednov of University of Texas at Austin.
This work was supported by funding from the National Institutes of Health (AA013498, AA027700, AA021491, AA017447, AA006420, T32-AA007456, AA029841, AA025408, AA017823, AA025996, GM129454, AA013520), The Schimmel Family Chair, The Pearson Center for Alcoholism and Addiction Research, and The Scripps Research Institute’s Animal Models Core Facility.
For more information, contact press@scripps.edu

