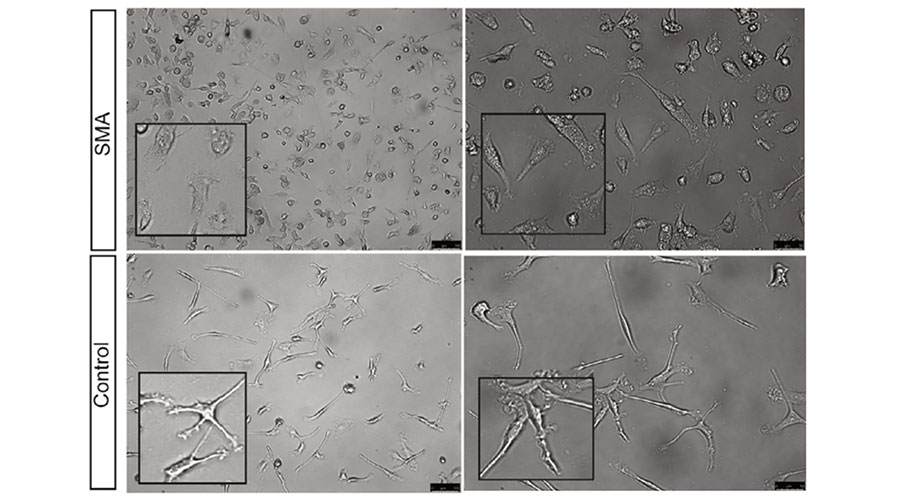
Microglia differentiated from iPSCs obtained from patients with SMA appeared differently from the control microglia, resembling an irregular, amoeba-like shape. Credit: Calibr and Uniformed Services University of the Health Sciences
‘Scavenger’ cells in the nervous system linked to rare genetic disease
Calibr, the drug development arm of Scripps Research, and other collaborators find microglia play a critical role in SMA disease progression.
September 30, 2022
LA JOLLA, CA—Spinal muscular atrophy (SMA) is a devastating, rare genetic disease that causes muscle weakness and eventually atrophy all throughout the body. Scientists at Calibr, the drug development arm of Scripps Research, and additional partners have now identified how a critical immune cell—known as the microglia—impacts SMA.
Published in Glia in July 2022, the findings provide a foundational understanding of SMA and how the interconnected neuro-immune network influences the disease. These insights could help lead to more effective medicines that target the newly discovered pathways.
“While there are three drugs currently on the market to treat SMA, they can be toxic and aren’t completely effective at alleviating the life-threatening nature of the disease,” says co-first author Zaida A. Gloria, a principal scientist at Calibr. “As our discoveries indicate, we’re beginning to understand the reason is because of just how multi-faceted the disease is. There’s now promise in creating medicines that target these additional pathways, while improving lifespan and overall quality of life for patients.”
SMA is characterized by a deletion or mutation in SMN1, the gene that produces the survival motor neuron (SMN) protein. Without this protein, the muscles that control activities like breathing, swallowing and walking begin to weaken. If left untreated, most patients with SMA don’t survive past two years of age.
While SMA research has historically always centered on enhancing SMN protein expression, the researchers were interested in the supportive roles of other immune cells instead, such as the microglia. The microglia are cells responsible for ‘eating up’ dying cells and debris (a process called phagocytosis); migrating around the central nervous system; and sending chemical messengers to recruit additional helper cells to injury sites.
“The microglia can be thought of as the nervous system’s scavengers and surveyors—they call out to the immune system if something is wrong or needs more attention,” says co-first author Guzal Khayrullina, PhD, Uniformed Services University of the Health Sciences (USUHS) neuroscience graduate. “We wanted to understand how reduced levels of SMN impacts this cell, as very limited research has specifically focused on this question before.”
In the study, the researchers utilized mice with low levels of SMN protein, as well as induced pluripotent stem cells (iPSCs) taken from patients with SMA. iPSCs are made by taking a person’s skin or blood cells and then reprogramming them back into their earlier, native state—before they’ve become specialized into a specific cell type. This was an important component of the study, as there is not much work focusing on microglia differentiated from SMA patient-derived iPSCs.
In SMN-deficient mice, the team found the microglia in the lumbar region of the spinal cord have a rounded shape with reduced processes, which suggests these cells are in an activated state. By studying the microglia differentiated from SMA-patient iPSCs, they also saw that the SMN-deficient microglia had increased cell migration and phagocytosis. Taken together, these activities could potentially impact the SMA disease pathology overall.
“These discoveries solidified our theory that SMA is not just a disease of motor neurons and is likely influenced by multiple different cell and tissue types,” says senior author Barrington Burnett, PhD, associate professor and vice chair for research at USUHS. “We can now explore how the SMN protein regulates the communications between microglia and motor neurons and the contributions of this interaction to the disease progression, and as a result, develop additional therapies that can make currently available treatment options even more effective.”
The researchers plan to further examine the complex nervous and immune system networks involved in SMA, as well as study an additional brain cell type called astrocytes shown to be implicated in the disease.
The study was funded by the Vanier CIHR Doctoral Research Award; Frederick Banting and Charles Best CIHR Doctoral Research Award; Canadian Institutes of Health Research; and the National Institute of Neurological Disorders and Stroke.
In addition to Gloria, Khayrullina and Burnett, authors of the study, “Survival motor neuron protein deficiency alters microglia reactivity,” included Kristen A. Johnson of Calibr; Matthew Stinson, Natalya Belous, Elizabeth M. Bergman, Fritz W. Lischka, Jeremy Rotty and Clifton L. Dalgard of the Uniformed Services University of the Health Sciences; and Marc-Olivier Deguise, Sabrina Gagnon, Lucia Chehade and Rashmi Kothary of Ottawa Hospital Research Institute and the University of Ottawa.
For more information, contact press@scripps.edu

