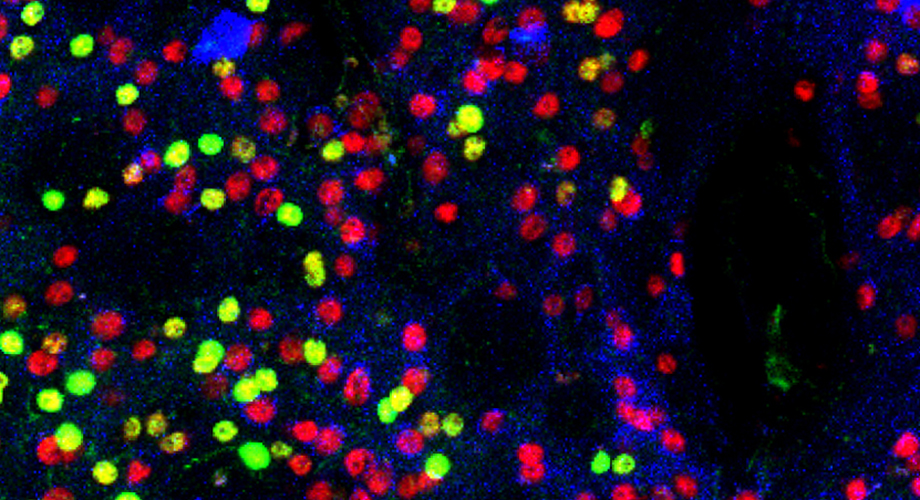
Mouse neurons from the striatum are tagged with fluorescent dye to show the presence of the mTOR protein. Those in yellow and green are missing the protein. (Photo courtesy of Subramaniam lab.)
Dangerous side effect of widely used psychiatric drug may be treatable with existing medicine
Scripps Research scientists identify a medicine that prevents and treats a severe reaction to the drug haloperidol, also known as Haldol, commonly used for schizophrenia and bi-polar disorder.
October 07, 2020
JUPITER, FL—The widely used psychiatric drug haloperidol, also known as Haldol, can cause a life-threatening adverse event called catalepsy, which leaves patients in a trance-like state accompanied by rigid muscles and abnormal, uncontrollable movements. There is no antidote. But an unexpected finding from scientists at Scripps Research may change that.

Srinivasa Subramaniam, PhD
The researchers discovered an underlying cause of haloperidol-induced catalepsy in a region of the brain called the striatum, and identified another existing medication that, in animal studies, prevents and rescues the condition.
Their study, published in the journal Translational Psychiatry, illuminates an aspect of neuronal signaling in the brain’s striatum that is important in epilepsy, Parkinson’s disease, Huntington’s disease and other conditions, says lead study author Srinivasa Subramaniam, PhD, associate professor of Neuroscience on Scripps Research’s Jupiter, Florida, campus. Specifically, it shows how a key protein involved in dopamine 2 signaling, called mTOR, can lead to a variety of serious disorders if it is over- or under-expressed in the striatum, he explains.
Equally important, the study suggests a readily available medication used to coat heart stents and prevent rejection of transplanted organs prevents or rescues haloperidol-induced catalepsy in mouse studies. The drug, rapamycin, also known as sirolimus, protected mice in haloperidol-induced catalepsy in a dose-dependent manner, Subramaniam says.
“Further study of the potential for rapamycin to benefit patients in this unfortunate situation may be warranted based on the results of our studies,” Subramaniam says.
It was an unexpected finding for the researchers, who had not set out to search for an antidote for haloperidol-induced catalepsy.
“Our lab works to understand disease mechanisms based in specific areas of the brain. During our study of the striatum, we used known chemical tools to probe, isolate and modulate key proteins and circuits,” Subramaniam says. “Haloperidol and rapamycin were among the chemical tools we used. In experiment after experiment, we saw clearly that mice experiencing haloperidol-induced catalepsy or mTOR-silenced catalepsy were protected by rapamycin. In the medical literature this condition in humans is described as untreatable, so we felt compelled to report these results to the wider scientific community as soon as possible.”
Haloperidol is an anti-psychotic and mood stabilizing medication that is inexpensive and widely used for people with conditions including schizophrenia and bi-polar disorder. It works in part by blocking the receptor for the neurotransmitter dopamine 2, involved in motor, motivational and reward-seeking behavior, in coordination with dopamine 1.
While haloperidol helps many people with serious mental health conditions, its list of potential side-effects and adverse events is long. For example, it has been found to increase risk of sudden heart failure and death in dementia patients, leading the U.S Food and Drug Administration to issue a black-box warning against its prescription to manage agitation in seniors with conditions including Alzheimer’s.
The striatum is a brain region that acts a bit like a home’s electrical switch box, routing instructions about reward and movement to the basal ganglia, which activates movement. In the striatum, mTOR acts in concert with a complex of proteins called mTORC1 to influence dopamine signaling. It must operate within a narrow, healthy range, Subramaniam says. Too much or too little has been linked to epilepsy, intellectual disability, tuberous sclerosis, Huntington disease, Parkinson’s disease and Alzheimer’s, depending on specific neurons affected.
While much has been learned about the role of mTOR in orchestrating diverse functions ranging from embryonic development to aging, its role independently in neuron signaling within the brain’s striatum was not previously understood, Subramaniam says.
“Both genetic depletion of mTOR or pharmacological inhibition of mTORC1 signaling by rapamycin prevented a haloperidol-induced catalepsy response,” Subramaniam says. “These findings may be important to help prevent severe adverse events of haloperidol and help patients in this difficult situation.”
The study, “The mammalian target of rapamycin (mTOR) kinase mediates haloperidol-induced cataleptic behavior,” appears online October 2, 2020 in the journal Translational Psychiatry. In addition to lead author Subramanim, authors include first author Uri Nimrod Ramírez-Jarquín, Neelam Shahani and William Pryor of Scripps Research in Jupiter, Florida, and Alessandro Usiello of the University of Campania Luigi Vanvitelli in Caserta Italy and the Laboratory of Behavioral Neuroscience, Ceinge Biotecnologie Avanzate, in Naples, Italy.
The research was supported by funding from the National Institutes of Health/National Institute of Neurological Disorders and Stroke and a grant from Cure Huntington Disease Initiative foundation.
For more information, contact press@scripps.edu

