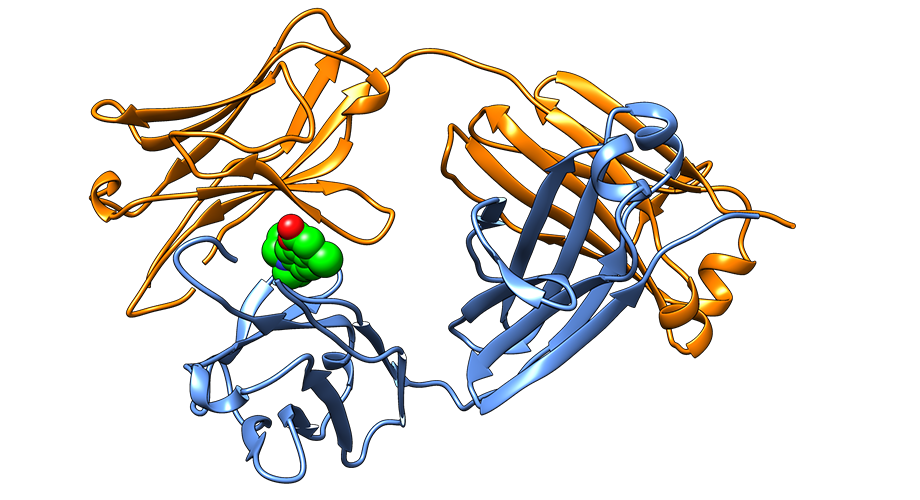
Crystal structure of the native state of a light chain protein whose aggregation causes AL amyloidosis, successfully stabilized by a small molecule identified in the Kelly Lab.
Molecules that prevent protein misfolding point to new type of therapy for AL amyloidosis
Scripps Research team finds promising molecules for stabilizing abnormal proteins that cause disease
April 11, 2019
LA JOLLA, CA – Scientists at Scripps Research have identified a group of small molecules that prevent structural changes to proteins that are at the root of AL amyloidosis, also called light chain amyloidosis, a progressive and often fatal disease.
The small molecules bind to and stabilize immunoglobulin light chain proteins, which, in AL amyloidosis, are secreted as isolated light chain dimers instead of components of antibodies comprising the immune system. Stabilizing the light chains in their native shape prevents them from misfolding and forming the toxic plaques found in patients with AL amyloidosis.
By labeling light chains with fluorophores and coupling shape changes to cleavage by proteinase K, nearly a million small molecules were screened for their ability to prevent the disease-associated structural changes using fluorescence polarization. Using this strategy as the basis for a high-throughput screen and various distinct counter-screens to eliminate artifacts, the team discovered multiple small-molecule drug candidates that prevented immunoglobulin light chains from misfolding and aggregating in a test tube. The approach, if successful in humans, could change the course of AL amyloidosis.
“Because we have characterized the immunoglobulin light chain small molecule binding site conferring stabilization by crystallography, we believe these findings represent a blueprint for making a much-needed drug for AL amyloidosis,” says Scripps Research chemistry professor Jeffery Kelly, PhD, who led the research. “If we can block the aggregation of newly secreted light chains and prevent them from being degraded into aggregation-prone fragments, we hope to slow or even halt disease progression, especially for those patients exhibiting cardiac involvement.”
Amyloidosis is a rare and likely underdiagnosed disease that takes many forms. AL amyloidosis is the second most common systemic amyloid disease, with some 4,500 new cases every year in the U.S.
The plasma cells of people with AL amyloidosis produce a component of antibodies known as immunoglobulin light chains, often instead of antibodies, as a result of a plasma cell cancer. The light chains misfold and/or clump together into small fibers that are toxic to organs. Over time, these so-called amyloid deposits progressively interfere with healthy function of the heart, kidneys, liver and other parts of the body.
Gareth Morgan, PhD, lead author of the study, emphasizes that AL amyloidosis patients with organ involvement, particularly cardiac involvement, are often too sick to tolerate chemotherapy. The hope is that immunoglobulin light chain kinetic stabilizers identified in this study will be able to serve as a first treatment for these patients so that they can ultimately tolerate chemotherapy.
The Scripps Research team’s findings appear in the latest issue of the Proceedings of the National Academy of Sciences. In addition to describing their kinetic stabilization approach for treating AL amyloidosis, the researchers say they envision a straightforward method for identifying the patients who would be most likely to benefit from the treatment.
Kelly says the drug mechanism that the team identified is analogous to the mechanism underpinning a different drug that also originated at Scripps Research: Tafamidis, which stabilizes the protein transthyretin to treat the most common systemic amyloid disease that affects the heart and/or other organs. Kelly invented tafamidis along with Evan Powers, PhD, to treat transthyretin amyloidoses. Now owned by Pfizer, tafamidis is approved in Europe under the name Vyndaqel and was recently submitted to the FDA for approval in the U.S.
Nicholas Yan, a graduate student and co-first author on the study, is now developing more potent and selective immunoglobulin light chain kinetic stabilizers with drug-like properties to be used in humans, studies being carried out in collaboration with Reji Nair, PhD, a staff scientist, also in collaboration with others at Scripps Research.
Additional authors of the study, “Stabilization of amyloidogenic immunoglobulin light chains by small molecules,” were Nicholas L. Yan, David E. Mortenson, Joshua M. Blundon, Ryan M. Gwin, Chung-Yon Lin, Steven J. Brown, Hugh Rosen, Jeffery W. Kelly, Ian A. Wilson, Robyn L. Stanfield, Timothy P. Spicer, Virneliz Fernandez-Vega of Scripps Research; Lewis E. Kay from The Hospital for Sick Children; Enrico Rennella and Lewis E. Kay from The University of Toronto; Giampaolo Merlini from University of Pavia; and Giampaolo Merlini from Foundation Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo.
This study was supported by the National Institutes of Health (grants DK46335 and UL1TR002550), the Skaggs Institute for Chemical Biology, and the Lita Annenberg Hazen Foundation.
For more information, contact press@scripps.edu

