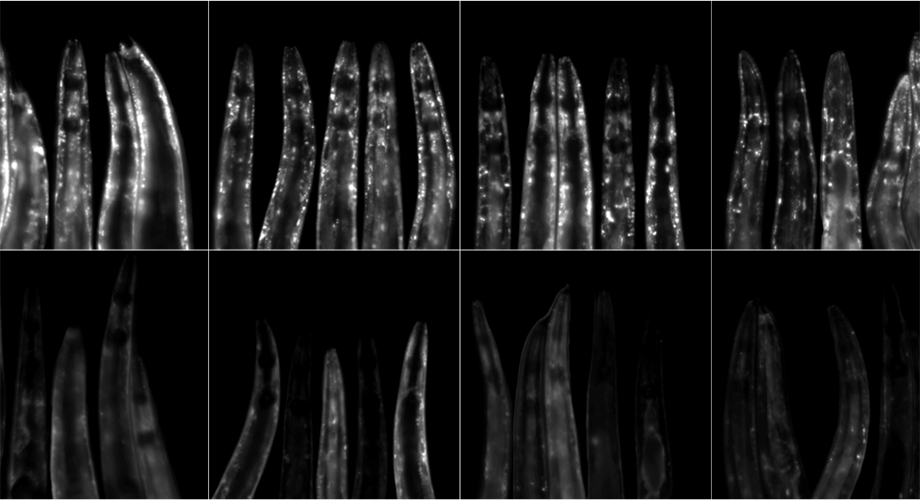
This image shows C. elegans roundworms expressing the a-synuclein::YFP (white spots), a protein known to build up in neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease. The worms on the bottom row were given the antibiotic minocycline and have less age-associated buildup of the protein compared to the worms on the top row, which received no minocycline. Credit: Gregory Solis/Michael Petrascheck
Antibiotic could protect against neurodegenerative diseases during aging
November 28, 2018
La Jolla, CA – The antibiotic minocycline increases the lifespan of aged roundworms and prevents the buildup of proteins, revealing a protective mechanism that could be exploited therapeutically to help prevent neurodegenerative diseases.

Now, scientists at Scripps Research have found that minocycline can increase the lifespan of roundworms by preventing the buildup of proteins during aging, according to a study in the open-access journal eLife reports.
Protein aggregation causes several progressive age-related brain diseases, including amyotrophic lateral sclerosis, Alzheimer's, Parkinson's and prion diseases. The Scripps Research study shows that minocycline prevents this buildup even in older animals with age-impaired stress-response pathways.
The number of proteins in a cell is balanced by the rate of protein manufacture and disposal, called proteostasis. As we age, proteostasis becomes impaired.
“It would be great if there were a way to enhance proteostasis and extend lifespan and health, by treating older people at the first sign of neurodegenerative symptoms or disease markers such as protein build-up,” says lead author Gregory Solis, a graduate student at Scripps Research. “In this study, we investigated whether minocycline can reduce protein aggregation and extend lifespan in animals that already have impaired proteostasis.”
The team first tested 21 different molecules known to extend lifespan in young and old Caenorhabditis elegans (C. elegans) worms. They found that all of these molecules prolonged the lives of young worms, however, the only drug that worked on the older worms was minocycline.
To find out why, the researchers looked at whether minocycline had any effect on protein aggregation in the worms. They treated young and old worms with water or minocycline and then measured two proteins, α-synuclein and amyloid-β, which are known to build up in Parkinson’s and Alzheimer’s, respectively. Regardless of the worms’ age, those treated with minocycline had reduced aggregation of both proteins as they grew older.
The team next turned their attention to the mechanism behind this discovery. First, they looked at whether minocycline switches on stress-signaling proteins that are impaired in older worms, but they found the drug actually reduces their activity. Next, they studied whether it turns off the cell’s protein-disposal processes, but this was not its mode of action either.
When researchers used a chemical probe to see how minocycline affects the major protein-regulating molecules in the cell, it revealed that minocycline directly affects the protein-manufacturing machinery of the cell, known as the ribosome. This was true in worms, as well as in mouse and human cells.
Finally, the team used worms with increased or decreased protein-manufacturing activity and studied how this altered the effect of minocycline on protein levels and lifespan. As predicted, in mutant worms where protein manufacturing was already decreased, they found a lower dose of minocycline was needed to further reduce protein levels and extend lifespan. In worms where protein manufacturing was increased, the opposite was seen. This suggested that minocycline extends lifespan by controlling the rate of protein manufacturing at the ribosome.
“We have identified minocycline as a drug that can extend lifespan and improve protein balance in already-aging worms,” says Michael Petrascheck, PhD, senior author of the paper and associate professor at Scripps Research. “Our study reveals how minocycline prevents protein aggregation and lays the foundations for drug development efforts aimed at optimizing this already-approved drug for a range of neurodegenerative diseases.”
Other authors on “Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms,” include Gregory M. Solis, Rozina Kardakaris, Elizabeth R.Valentine, Liron Bar-Peled, Alice L. Chen, Megan M. Blewett, Mark A. McCormick, James R. Williamson, Brian K. Kennedy and Benjamin F. Cravatt, of Scripps Research.
The study was supported by the National Institutes of Health (grant DP2 OD008398, grant R21NS107951), the Ellison Foundation (AG-NS-0928-12), the Glenn Foundation for Medical Research and the National Science Foundation (NSF/DGE-1346837).
For more information, contact press@scripps.edu

