Scientists Develop Broad-Spectrum Inhibitors of Influenza Viruses
A team of researchers from The Scripps Research Institute (TSRI) and Janssen Research & Development (Janssen) has devised artificial peptide molecules that neutralize a broad range of influenza virus strains. Peptides are short chains of amino acids – like proteins but with smaller, simpler structures. These designed molecules have the potential to be developed into medicines that target influenza, which causes up to 500,000 deaths worldwide each year[i] and costs Americans billions of dollars in sick days and lost productivity.[ii]
The developed peptides block the infectivity of most circulating strains of group 1 influenza A viruses, including H5N1, an avian flu strain that has caused hundreds of human infections and deaths in Asia, and the H1N1 swine flu strain that caused a global pandemic in 2009-10.
The scientists designed the peptides to mimic the virus-gripping regions of two recently discovered “super-antibodies” that are known to neutralize virtually all influenza A strains. Antibodies are large proteins that are expensive to produce and must be delivered by injection or infusion. Whereas, “the peptides developed in the study have the potential to be medicines delivered via pill-based drugs in the future.”
“Making small molecules that do essentially what these larger, broadly neutralizing antibodies do is a really exciting and promising strategy against influenza, as our new results show,” said co-senior investigator Ian Wilson, Hansen Professor of Structural Biology at TSRI.
The report on the new peptides appeared as an online First Release paper in Science on September 28, 2017.
The two anti-flu super-antibodies on which these peptides are based, called FI6v3 and CR9114, were discovered in 2011 and 2012. Since then, Wilson’s laboratory at TSRI in partnership with Janssen and other structural biology laboratories around the world have mapped at atomic scale how these and other broadly neutralizing antibodies bind to flu viruses.
A research team led by David Baker at the University of Washington recently used these antibody-structure data to design novel proteins, smaller than the antibodies, that bind to flu viruses in a similar way and neutralize a broad range of flu strains. The new effort by TSRI in collaboration with Janssen scientists aimed at the development of even smaller non-protein-like molecules that would hit the same target region on flu viruses.
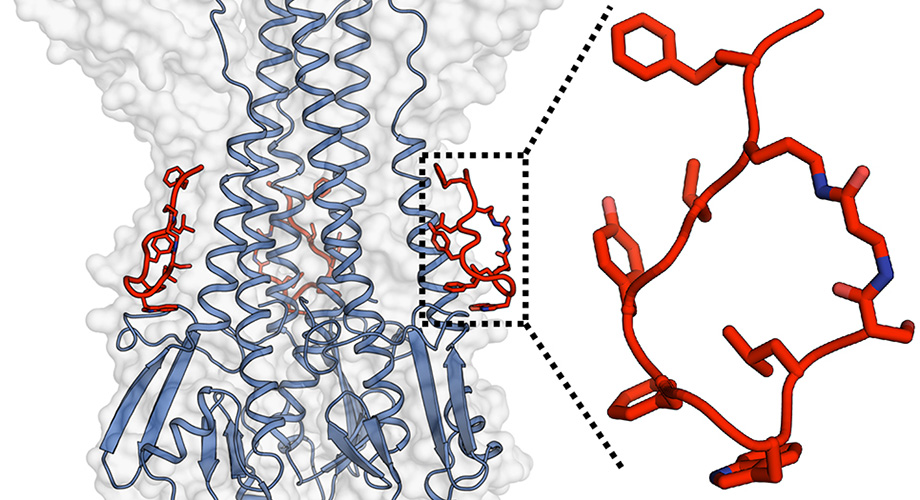
Following several rounds of molecular design and synthesis, virus-binding testing, and atomic-level structural evaluation, the research team developed a set of four peptides with circular, “cyclic” structures that performed well as potential flu-blocking molecules.
The peptides showed high binding affinity for a broad set of group 1 influenza A viruses, as well as a potent ability to neutralize infections with these viruses in the laboratory experiments. The targeted group 1 influenza A viruses include H1, H2, H5 and H6 subtypes.
The peptides also incorporated amino-acid building blocks that are not found in natural proteins, and this, as well as their cyclic structures, made them relatively resistant to the enzymes that can otherwise quickly clear peptide drugs from the bloodstream. The most optimized of the four peptides, named P7, survived for hours when exposed to mouse or human blood plasma, or when injected into mice.
“These peptides have drug-like stability and will be good candidates for further testing of antiviral efficacy in animal models,” said Rameshwar U. Kadam, a senior postdoctoral research associate in the Wilson Laboratory who is co-first author of the study along with Jarek Juraszek, Principal Scientist at Janssen.
The peptides, like the antibodies they are designed to mimic, bind to a site known as the hydrophobic stem groove on the lower part of the flu virus’s main envelope protein hemagglutinin. The molecular structure at this site does not tend to vary much among flu strains because it plays a crucial role in a shape-shifting process that permits the virus to penetrate the host cell and initiate infection. Structural evaluations by Kadam found that the peptides prevent this shape-change and thus preventing host cell penetration.
“A therapy that targets the first stage of infection would complement the existing anti-influenza drugs that target later stages of infection,” Kadam said.
The peptides don’t bind to their viral target as comprehensively as the antibodies on which they are based. On group 2 influenza A viruses, for example, they lacked the bulkier antibodies’ ability to push aside or avoid a sugar molecule on the hemagglutinin that blocks a key part of the target site. However, Kadam said that further studies may yield peptides with activity against both group 1 and group 2 influenza A and even influenza B strains.
“It’s pretty revolutionary that we were able to use structural information on antibodies to make much smaller molecules that have almost the same binding affinity and breadth of neutralization against flu viruses,” said Kadam.
“There has been skepticism in the field that we could get such good results with such small molecules, but this study proves that we can,” Wilson said.
Other co-authors of the paper, “Potent Peptidic Fusion Inhibitors of Influenza Virus,” were Boerries Brandenburg, Christophe Buyck, Wim Schepens, Bart Kesteleyn, Bart Stoops, Rob Vreeken, Jan Vermond, Wouter Goutier, Chan Tang, Ronald Vogels, Robert Friesen, Jaap Goudsmit and co-senior investigator Maria van Dongen.
The research was supported by Janssen and the National Institutes of Health (R56 AI117675 and R56 AI127371).
[i] WHO Influenza Fact Sheet. http://www.who.int/mediacentre/factsheets/fs211/en/
[ii] The National Institute for Occupational Safety and Health (NIOSH) “Influenza in the Work Place” Fact Sheet. https://www.cdc.gov/niosh/topics/flu/activities.html
For more information, contact press@scripps.edu See More News

