Status 121-148
Experiments 143 to 148 (AutoDock), 0% completed
Experiments 143 through 148 target the LEDGF site of HIV integrase. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M and Maybridge). These experiments will help distinguish better sites of among a group of receptor structures even within the same structure. To help read structures in the table below, the "a" and "b" after the 4-character PDB ID designate those receptor structures with alternate residue positions, while the "a" and "b" with the sites (FBP, LEDGF, Y3) designate different sites for the same symmetric structure of HIV IN.
| AD Exp. 143 | AD Exp. 144 | AD Exp. 145 | AD Exp. 146 | AD Exp. 147 | AD Exp. 148 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 4GVMa, LEDGF Site 1 | 824077-824393 | 825345-827689 | 834725-835738 | 838781-839287 | 840809-842312 | 846825-846901 |
| PDB 4GVMb, LEDGF Site 1 | 824394-824710 | 827690-830034 | 835739-836752 | 839288-839794 | 842313-843816 | 846902-846978 |
| PDB 4GW6a, LEDGF Site 2 | 824711-825027 | 830035-832379 | 836753-837766 | 839795-840301 | 843817-845320 | 846979-847055 |
| PDB 4GW6b, LEDGF Site 2 | 825028-825344 | 832380-834724 | 837767-838780 | 840302-840808 | 845321-846824 | 847056-847132 |
| COMPLETION | 0% | 0% | 0% | 0% | 0% | 0% |
Experiments 139 to 142 (AD and AD Vina), 0% completed
Experiments 139 through 142 are two studies that each target the the combined receptors thus far used for AutoDock Vina. For each study, AutoDock and AD Vina screen either the NCI Diversity Set II* or the Full NCI Set; hence, there are 4 main groupings of virtual screenings. Experiments 139 and 140 will explore the calibration of a receptor when multiple receptors are involved in a virtual screening. Experiments 141 and 142 will investigate the effect of re-orienting the grid box by systematically rotating and translating a protein structure. In these cases, the LEDGF site of the HIV-1 integrase receptor from PDB 3NF8 is used, 1800 versions. *Note: the current version of the NCI Diversity Set is IV.
| AD Exp. 139 AutoDock | AD Exp. 140 AD Vina | AD Exp. 141 AutoDock | AD Exp. 142 AD Vina | |
|---|---|---|---|---|
| Receptor Structure | NCI Div. 2 | NCI Div. 2 | NCI Div. 2 | Full NCI |
| Current Vina Receptors | 761260-765337 | 762338-762876 | 762877-764676 | 764677-824076 |
| COMPLETION | 0% | 0% | 0% | 0% |
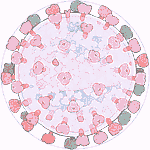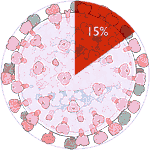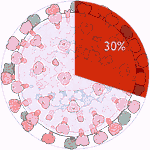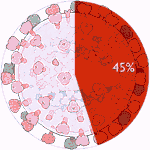
Experiments 133 to 138 (AD Vina), 45% completed
Experiments 133 through 138 target the dimer cavity (between the 2 LEDGF sites) of HIV integrase. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M and Maybridge). These experiments will help determine the differences and utility of varying the dimensions of a "docking box."
| AD Exp. 133 AD Vina Exp. 79 | AD Exp. 134 AD Vina Exp. 80 | AD Exp. 135 AD Vina Exp. 81 | AD Exp. 136 AD Vina Exp. 82 | AD Exp. 137 AD Vina Exp. 83 | AD Exp. 138 AD Vina Exp. 84 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 3AO1, dimer cavity | 732310-732341 | 733910-734144 | 745660-745761 | 750760-750810 | 753310-753460 | 760860-760867 |
| PDB 3AO2, dimer cavity | 732342-732373 | 734145-734379 | 745762-745863 | 750811-750861 | 753461-753611 | 760868-760875 |
| PDB 3AV9a, dimer cavity | 732374-732405 | 734380-734614 | 745864-745965 | 750862-750912 | 753612-753762 | 760876-760883 |
| PDB 3AV9b, dimer cavity | 732406-732437 | 734615-734849 | 745966-746067 | 750913-750963 | 753763-753913 | 760884-760891 |
| PDB 3AVAa, dimer cavity | 732438-732469 | 734850-735084 | 746068-746169 | 750964-751014 | 753914-754064 | 760892-760899 |
| PDB 3AVAb, dimer cavity | 732470-732501 | 735085-735319 | 746170-746271 | 751015-751065 | 754065-754215 | 760900-760907 |
| PDB 3AVCa, dimer cavity | 732502-732533 | 735320-735554 | 746272-746373 | 751066-751116 | 754216-754366 | 760908-760915 |
| PDB 3AVCb, dimer cavity | 732534-732565 | 735555-735789 | 746374-746475 | 751117-751167 | 754367-754517 | 760916-760923 |
| PDB 3AVFa, dimer cavity | 732566-732597 | 735790-736024 | 746476-746577 | 751168-751218 | 754518-754668 | 760924-760931 |
| PDB 3AVFb, dimer cavity | 732598-732629 | 736025-736259 | 746578-746679 | 751219-751269 | 754669-754819 | 760932-760939 |
| PDB 3AVGa, dimer cavity | 732630-732661 | 736260-736494 | 746680-746781 | 751270-751320 | 754820-754970 | 760940-760947 |
| PDB 3AVGb, dimer cavity | 732662-732693 | 736495-736729 | 746782-746883 | 751321-751371 | 754971-755121 | 760948-760955 |
| PDB 3AVHa, dimer cavity | 732694-732725 | 736730-736964 | 746884-746985 | 751372-751422 | 755122-755272 | 760956-760963 |
| PDB 3AVHb, dimer cavity | 732726-732757 | 736965-737199 | 746986-747087 | 751423-751473 | 755273-755423 | 760964-760971 |
| PDB 3AVIa, dimer cavity | 732758-732789 | 737200-737434 | 747088-747189 | 751474-751524 | 755424-755574 | 760972-760979 |
| PDB 3AVIb, dimer cavity | 732790-732821 | 737435-737669 | 747190-747291 | 751525-751575 | 755575-755725 | 760980-760987 |
| PDB 3AVJa, dimer cavity | 732822-732853 | 737670-737904 | 747292-747393 | 751576-751626 | 755726-755876 | 760988-760995 |
| PDB 3AVJb, dimer cavity | 732854-732885 | 737905-738139 | 747394-747495 | 751627-751677 | 755877-756027 | 760996-761003 |
| PDB 3AVKa, dimer cavity | 732886-732917 | 738140-738374 | 747496-747597 | 751678-751728 | 756028-756178 | 761004-761011 |
| PDB 3AVKb, dimer cavity | 732918-732949 | 738375-738609 | 747598-747699 | 751729-751779 | 756179-756329 | 761012-761019 |
| PDB 3AVLa, dimer cavity | 732950-732981 | 738610-738844 | 747700-747801 | 751780-751830 | 756330-756480 | 761020-761027 |
| PDB 3AVLb, dimer cavity | 732982-733013 | 738845-739079 | 747802-747903 | 751831-751881 | 756481-756631 | 761028-761035 |
| PDB 3AVMa, dimer cavity | 733014-733045 | 739080-739314 | 747904-748005 | 751882-751932 | 756632-756782 | 761036-761043 |
| PDB 3AVMb, dimer cavity | 733046-733077 | 739315-739549 | 748006-748107 | 751933-751983 | 756783-756933 | 761044-761051 |
| PDB 3AVNa, dimer cavity | 733078-733109 | 739550-739784 | 748108-748209 | 751984-752034 | 756934-757084 | 761052-761059 |
| PDB 3AVNb, dimer cavity | 733110-733141 | 739785-740019 | 748210-748311 | 752035-752085 | 757085-757235 | 761060-761067 |
| PDB 3NF6, dimer cavity | 733142-733173 | 740020-740254 | 748312-748413 | 752086-752136 | 757236-757386 | 761068-761075 |
| PDB 3NF7, dimer cavity | 733174-733205 | 740255-740489 | 748414-748515 | 752137-752187 | 757387-757537 | 761076-761083 |
| PDB 3NF8, dimer cavity | 733206-733237 | 740490-740724 | 748516-748617 | 752188-752238 | 757538-757688 | 761084-761091 |
| PDB 3NF9, dimer cavity | 733238-733269 | 740725-740959 | 748618-748719 | 752239-752289 | 757689-757839 | 761092-761099 |
| PDB 3NFA, dimer cavity | 733270-733301 | 740960-741194 | 748720-748821 | 752290-752340 | 757840-757990 | 761100-761107 |
| PDB 3VQ4, dimer cavity | 733302-733333 | 741195-741429 | 748822-748923 | 752341-752391 | 757991-758141 | 761108-761115 |
| PDB 3VQ5, dimer cavity | 733334-733365 | 741430-741664 | 748924-749025 | 752392-752442 | 758142-758292 | 761116-761123 |
| PDB 3VQ7, dimer cavity | 733366-733397 | 741665-741899 | 749026-749127 | 752443-752493 | 758293-758443 | 761124-761131 |
| PDB 3VQ8, dimer cavity | 733398-733429 | 741900-742134 | 749128-749229 | 752494-752544 | 758444-758594 | 761132-761139 |
| PDB 3VQA, dimer cavity | 733430-733461 | 742135-742369 | 749230-749331 | 752545-752595 | 758595-758745 | 761140-761147 |
| PDB 3VQD, dimer cavity | 733462-733493 | 742370-742604 | 749332-749433 | 752596-752646 | 758746-758896 | 761148-761155 |
| PDB 3VQE, dimer cavity | 733494-733525 | 742605-742839 | 749434-749535 | 752647-752697 | 758897-759047 | 761156-761163 |
| PDB 3ZCM, dimer cavity | 733526-733557 | 742840-743074 | 749536-749637 | 752698-752748 | 759048-759198 | 761164-761171 |
| PDB 3ZSO, dimer cavity | 733558-733589 | 743075-743309 | 749638-749739 | 752749-752799 | 759199-759349 | 761172-761179 |
| PDB 3ZSW, dimer cavity | 733590-733621 | 743310-743544 | 749740-749841 | 752800-752850 | 759350-759500 | 761180-761187 |
| PDB 3ZT1, dimer cavity | 733622-733653 | 743545-743779 | 749842-749943 | 752851-752901 | 759501-759651 | 761188-761195 |
| PDB 3ZT2, dimer cavity | 733654-733685 | 743780-744014 | 749944-750045 | 752902-752952 | 759652-759802 | 761196-761203 |
| PDB 3ZT3, dimer cavity | 733686-733717 | 744015-744249 | 750046-750147 | 752953-753003 | 759803-759953 | 761204-761211 |
| PDB 3ZT4, dimer cavity | 733718-733749 | 744250-744484 | 750148-750249 | 753004-753054 | 759954-760104 | 761212-761219 |
| PDB 4AHR, dimer cavity | 733750-733781 | 744485-744719 | 750250-750351 | 753055-753105 | 760105-760255 | 761220-761227 |
| PDB 4GVMa, dimer cavity | 733782-733813 | 744720-744954 | 750352-750453 | 753106-753156 | 760256-760406 | 761228-761235 |
| PDB 4GVMb, dimer cavity | 733814-733845 | 744955-745189 | 750454-750555 | 753157-753207 | 760407-760557 | 761236-761243 |
| PDB 4GW6a, dimer cavity | 733846-733877 | 745190-745424 | 750556-750657 | 753208-753258 | 760558-760708 | 761244-761251 |
| PDB 4GW6b, dimer cavity | 733878-733909 | 745425-745659 | 750658-750759 | 753259-753309 | 760709-760859 | 761252-761259 |
| COMPLETION | 4% | 95% | 99.9% | 74% | 0% | 0% |
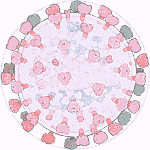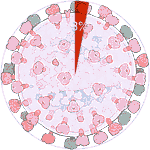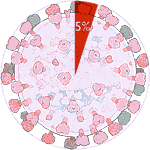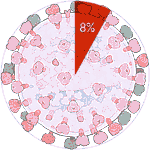
Experiments 127 to 132 (AD Vina), 8% completed
Experiments 127 through 132 target HIV reverse transcriptase. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M and Maybridge). These experiments will help determine the differences and utility of varying the dimensions of a "docking box."
| AD Exp. 127 AD Vina Exp. 73 | AD Exp. 128 AD Vina Exp. 74 | AD Exp. 129 AD Vina Exp. 75 | AD Exp. 130 AD Vina Exp. 76 | AD Exp. 131 AD Vina Exp. 77 | AD Exp. 132 AD Vina Exp. 78 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 1rt1, NNRTIadjacent site | 700465-700496 | 702225-702459 | 715150-715251 | 720760-720810 | 723565-723715 | 731870-731877 |
| PDB 1rt1, all sites | 700497-700528 | 702460-702694 | 715252-715353 | 720811-720861 | 723716-723866 | 731878-731885 |
| PDB 1rt1, Knuckles site | 700529-700560 | 702695-702929 | 715354-715455 | 720862-720912 | 723867-724017 | 731886-731893 |
| PDB 1rt1, Incoming Nucleotide Binding site | 700561-700592 | 702930-703164 | 715456-715557 | 720913-720963 | 724018-724168 | 731894-731901 |
| PDB 1rt1, NNRTI site | 700593-700624 | 703165-703399 | 715558-715659 | 720964-721014 | 724169-724319 | 731902-731909 |
| PDB 1rth, NNRTIadjacent site | 700625-700656 | 703400-703634 | 715660-715761 | 721015-721065 | 724320-724470 | 731910-731917 |
| PDB 1rth, all sites | 700657-700688 | 703635-703869 | 715762-715863 | 721066-721116 | 724471-724621 | 731918-731925 |
| PDB 1rth, Knuckles site | 700689-700720 | 703870-704104 | 715864-715965 | 721117-721167 | 724622-724772 | 731926-731933 |
| PDB 1rth, Incoming Nucleotide Binding site | 700721-700752 | 704105-704339 | 715966-716067 | 721168-721218 | 724773-724923 | 731934-731941 |
| PDB 1rth, NNRTI site | 700753-700784 | 704340-704574 | 716068-716169 | 721219-721269 | 724924-725074 | 731942-731949 |
| PDB 1vrt, NNRTIadjacent site | 700785-700816 | 704575-704809 | 716170-716271 | 721270-721320 | 725075-725225 | 731950-731957 |
| PDB 1vrt, all sites | 700817-700848 | 704810-705044 | 716272-716373 | 721321-721371 | 725226-725376 | 731958-731965 |
| PDB 1vrt, Knuckles site | 700849-700880 | 705045-705279 | 716374-716475 | 721372-721422 | 725377-725527 | 731966-731973 |
| PDB 1vrt, Incoming Nucleotide Binding site | 700881-700912 | 705280-705514 | 716476-716577 | 721423-721473 | 725528-725678 | 731974-731981 |
| PDB 1vrt, NNRTI site | 700913-700944 | 705515-705749 | 716578-716679 | 721474-721524 | 725679-725829 | 731982-731989 |
| PDB 1vru, NNRTIadjacent site | 700945-700976 | 705750-705984 | 716680-716781 | 721525-721575 | 725830-725980 | 731990-731997 |
| PDB 1vru, all sites | 700977-701008 | 705985-706219 | 716782-716883 | 721576-721626 | 725981-726131 | 731998-732005 |
| PDB 1vru, Knuckles site | 701009-701040 | 706220-706454 | 716884-716985 | 721627-721677 | 726132-726282 | 732006-732013 |
| PDB 1vru, Incoming Nucleotide Binding site | 701041-701072 | 706455-706689 | 716986-717087 | 721678-721728 | 726283-726433 | 732014-732021 |
| PDB 1vru, NNRTI site | 701073-701104 | 706690-706924 | 717088-717189 | 721729-721779 | 726434-726584 | 732022-732029 |
| PDB 2zd1, NNRTIadjacent site | 701105-701136 | 706925-707159 | 717190-717291 | 721780-721830 | 726585-726735 | 732030-732037 |
| PDB 2zd1, all sites | 701137-701168 | 707160-707394 | 717292-717393 | 721831-721881 | 726736-726886 | 732038-732045 |
| PDB 2zd1, Knuckles site | 701169-701200 | 707395-707629 | 717394-717495 | 721882-721932 | 726887-727037 | 732046-732053 |
| PDB 2zd1, Incoming Nucleotide Binding site | 701201-701232 | 707630-707864 | 717496-717597 | 721933-721983 | 727038-727188 | 732054-732061 |
| PDB 2zd1, NNRTI site | 701233-701264 | 707865-708099 | 717598-717699 | 721984-722034 | 727189-727339 | 732062-732069 |
| PDB 3jytNoMg, NNRTIadjacent site | 701265-701296 | 708100-708334 | 717700-717801 | 722035-722085 | 727340-727490 | 732070-732077 |
| PDB 3jytNoMg, all sites | 701297-701328 | 708335-708569 | 717802-717903 | 722086-722136 | 727491-727641 | 732078-732085 |
| PDB 3jytNoMg, Knuckles site | 701329-701360 | 708570-708804 | 717904-718005 | 722137-722187 | 727642-727792 | 732086-732093 |
| PDB 3jytNoMg, Incoming Nucleotide Binding site | 701361-701392 | 708805-709039 | 718006-718107 | 722188-722238 | 727793-727943 | 732094-732101 |
| PDB 3jytNoMg, NNRTI site | 701393-701424 | 709040-709274 | 718108-718209 | 722239-722289 | 727944-728094 | 732102-732109 |
| PDB 3jyt, NNRTIadjacent site | 701425-701456 | 709275-709509 | 718210-718311 | 722290-722340 | 728095-728245 | 732110-732117 |
| PDB 3jyt, all sites | 701457-701488 | 709510-709744 | 718312-718413 | 722341-722391 | 728246-728396 | 732118-732125 |
| PDB 3jyt, Knuckles site | 701489-701520 | 709745-709979 | 718414-718515 | 722392-722442 | 728397-728547 | 732126-732133 |
| PDB 3jyt, Incoming Nucleotide Binding site | 701521-701552 | 709980-710214 | 718516-718617 | 722443-722493 | 728548-728698 | 732134-732141 |
| PDB 3jyt, NNRTI site | 701553-701584 | 710215-710449 | 718618-718719 | 722494-722544 | 728699-728849 | 732142-732149 |
| PDB 4i2q, NNRTIadjacent site | 701585-701616 | 710450-710684 | 718720-718821 | 722545-722595 | 728850-729000 | 732150-732157 |
| PDB 4i2q, all sites | 701617-701648 | 710685-710919 | 718822-718923 | 722596-722646 | 729001-729151 | 732158-732165 |
| PDB 4i2q, Knuckles site | 701649-701680 | 710920-711154 | 718924-719025 | 722647-722697 | 729152-729302 | 732166-732173 |
| PDB 4i2q, Incoming Nucleotide Binding site | 701681-701712 | 711155-711389 | 719026-719127 | 722698-722748 | 729303-729453 | 732174-732181 |
| PDB 4i2q, NNRTI site | 701713-701744 | 711390-711624 | 719128-719229 | 722749-722799 | 729454-729604 | 732182-732189 |
| PDB 4icl, NNRTIadjacent site | 701745-701776 | 711625-711859 | 719230-719331 | 722800-722850 | 729605-729755 | 732190-732197 |
| PDB 4icl, all sites | 701777-701808 | 711860-712094 | 719332-719433 | 722851-722901 | 729756-729906 | 732198-732205 |
| PDB 4icl, Knuckles site | 701809-701840 | 712095-712329 | 719434-719535 | 722902-722952 | 729907-730057 | 732206-732213 |
| PDB 4icl, Incoming Nucleotide Binding site | 701841-701872 | 712330-712564 | 719536-719637 | 722953-723003 | 730058-730208 | 732214-732221 |
| PDB 4icl, NNRTI site | 701873-701904 | 712565-712799 | 719638-719739 | 723004-723054 | 730209-730359 | 732222-732229 |
| PDB 4ify, NNRTIadjacent site | 701905-701936 | 712800-713034 | 719740-719841 | 723055-723105 | 730360-730510 | 732230-732237 |
| PDB 4ify, all sites | 701937-701968 | 713035-713269 | 719842-719943 | 723106-723156 | 730511-730661 | 732238-732245 |
| PDB 4ify, Knuckles site | 701969-702000 | 713270-713504 | 719944-720045 | 723157-723207 | 730662-730812 | 732246-732253 |
| PDB 4ify, Incoming Nucleotide Binding site | 702001-702032 | 713505-713739 | 720046-720147 | 723208-723258 | 730813-730963 | 732254-732261 |
| PDB 4ify, NNRTI site | 702033-702064 | 713740-713974 | 720148-720249 | 723259-723309 | 730964-731114 | 732262-732269 |
| PDB 4kfb, NNRTIadjacent site | 702065-702096 | 713975-714209 | 720250-720351 | 723310-723360 | 731115-731265 | 732270-732277 |
| PDB 4kfb, all sites | 702097-702128 | 714210-714444 | 720352-720453 | 723361-723411 | 731266-731416 | 732278-732285 |
| PDB 4kfb, Knuckles site | 702129-702160 | 714445-714679 | 720454-720555 | 723412-723462 | 731417-731567 | 732286-732293 |
| PDB 4kfb, Incoming Nucleotide Binding site | 702161-702192 | 714680-714914 | 720556-720657 | 723463-723513 | 731568-731718 | 732294-732301 |
| PDB 4kfb, NNRTI site | 702193-702224 | 714915-715149 | 720658-720759 | 723514-723564 | 731719-731869 | 732302-732309 |
| COMPLETION | 0% | 0.2% | 2% | 2% | 13% | 30% |
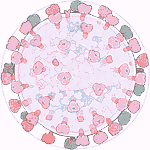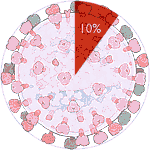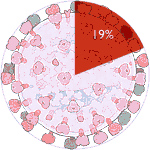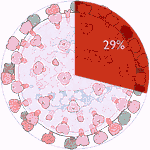
Experiments 121 to 126 (AD Vina), 29% completed
Experiments 121 through 126 target the active site of HIV protease. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M and Maybridge). These experiments will help determine the differences and utility of varying the dimensions of a "docking box." The focus of these experiments is on the allosteric sites of HIV protease.
| AD Exp. 121 AD Vina Exp. 67 | AD Exp. 122 AD Vina Exp. 68 | AD Exp. 123 AD Vina Exp. 69 | AD Exp. 124 AD Vina Exp. 70 | AD Exp. 125 AD Vina Exp. 71 | AD Exp. 126 AD Vina Exp. 72 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 1ZTZ, Exterior Sites, Side 1 | 552395-552426 | 560637-560871 | 621607-621708 | 647971-648021 | 661153-661303 | 700257-700264 |
| PDB 1ZTZ, Exterior Sites, Side 2 | 552427-552458 | 560872-561106 | 621709-621810 | 648022-648072 | 661304-661454 | 700265-700272 |
| PDB 3I8Wa, Exterior Sites, Side 1 | 552459-552490 | 561107-561341 | 621811-621912 | 648073-648123 | 661455-661605 | 700273-700280 |
| PDB 3I8Wa, Exterior Sites, Side 2 | 552491-552522 | 561342-561576 | 621913-622014 | 648124-648174 | 661606-661756 | 700281-700288 |
| PDB 3I8Wb, Exterior Sites, Side 1 | 552523-552554 | 561577-561811 | 622015-622116 | 648175-648225 | 661757-661907 | 700289-700296 |
| PDB 3I8Wb, Exterior Sites, Side 2 | 552555-552586 | 561812-562046 | 622117-622218 | 648226-648276 | 661908-662058 | 700297-700304 |
| PDB 3KF0, Exterior Sites, Side 1 | 552587-552618 | 562047-562281 | 622219-622320 | 648277-648327 | 662059-662209 | 700305-700312 |
| PDB 3KF0, Exterior Sites, Side 2 | 552619-552650 | 562282-562516 | 622321-622422 | 648328-648378 | 662210-662360 | 700313-700320 |
| PDB 3KFN, Exterior Sites, Side 1 | 552651-552682 | 562517-562751 | 622423-622524 | 648379-648429 | 662361-662511 | 700321-700328 |
| PDB 3KFN, Exterior Sites, Side 2 | 552683-552714 | 562752-562986 | 622525-622626 | 648430-648480 | 662512-662662 | 700329-700336 |
| PDB 3KFP, Exterior Sites, Side 1 | 552715-552746 | 562987-563221 | 622627-622728 | 648481-648531 | 662663-662813 | 700337-700344 |
| PDB 3KFP, Exterior Sites, Side 2 | 552747-552778 | 563222-563456 | 622729-622830 | 648532-648582 | 662814-662964 | 700345-700352 |
| PDB 3KFR, Exterior Sites, Side 1 | 552779-552810 | 563457-563691 | 622831-622932 | 648583-648633 | 662965-663115 | 700353-700360 |
| PDB 3KFR, Exterior Sites, Side 2 | 552811-552842 | 563692-563926 | 622933-623034 | 648634-648684 | 663116-663266 | 700361-700368 |
| PDB 3KFS, Exterior Sites, Side 1 | 552843-552874 | 563927-564161 | 623035-623136 | 648685-648735 | 663267-663417 | 700369-700376 |
| PDB 3KFS, Exterior Sites, Side 2 | 552875-552906 | 564162-564396 | 623137-623238 | 648736-648786 | 663418-663568 | 700377-700384 |
| PDB 3T11a, Exterior Sites, Side 1 | 552907-552938 | 564397-564631 | 623239-623340 | 648787-648837 | 663569-663719 | 700385-700392 |
| PDB 3T11a, Exterior Sites, Side 2 | 552939-552970 | 564632-564866 | 623341-623442 | 648838-648888 | 663720-663870 | 700393-700400 |
| PDB 3T11b, Exterior Sites, Side 1 | 552971-553002 | 564867-565101 | 623443-623544 | 648889-648939 | 663871-664021 | 700401-700408 |
| PDB 3T11b, Exterior Sites, Side 2 | 553003-553034 | 565102-565336 | 623545-623646 | 648940-648990 | 664022-664172 | 700409-700416 |
| PDB 4DQG, Exterior Sites, Side 1 | 553035-553066 | 565337-565571 | 623647-623748 | 648991-649041 | 664173-664323 | 700417-700424 |
| PDB 4DQG, Exterior Sites, Side 2 | 553067-553098 | 565572-565806 | 623749-623850 | 649042-649092 | 664324-664474 | 700425-700432 |
| PDB 4E43a, Exterior Sites, Side 1 | 553099-553130 | 565807-566041 | 623851-623952 | 649093-649143 | 664475-664625 | 700433-700440 |
| PDB 4E43a, Exterior Sites, Side 2 | 553131-553162 | 566042-566276 | 623953-624054 | 649144-649194 | 664626-664776 | 700441-700448 |
| PDB 4E43b, Exterior Sites, Side 1 | 553163-553194 | 566277-566511 | 624055-624156 | 649195-649245 | 664777-664927 | 700449-700456 |
| PDB 4E43b, Exterior Sites, Side 2 | 553195-553226 | 566512-566746 | 624157-624258 | 649246-649296 | 664928-665078 | 700457-700464 |
| COMPLETION | 0% | 0% | 0% | 91% | 84% | 0% |
Status 103-120
Experiments 115 to 120 (AD Vina), 79% completed
Experiments 115 through 120 target the active site of HIV protease. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M and Maybridge). These experiments will help distinguish better sites of among a group of receptor structures even within the same structure. Potentially important molecules (in these cases: water and/or chloride ion) found in the original structures are left in different combinations to produce varied models for HIV protease.
| AD Exp. 115 AD Vina Exp. 61 | AD Exp. 116 AD Vina Exp. 62 | AD Exp. 117 AD Vina Exp. 63 | AD Exp. 118 AD Vina Exp. 64 | AD Exp. 119 AD Vina Exp. 65 | AD Exp. 120 AD Vina Exp. 66 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 1ZTZ, Active Site | 396767-396798 | 405326-405560 | 468641-468742 | 496019-496069 | 509708-509858 | 550316-550323 |
| PDB 3I8Wa, Active Site | 396799-396830 | 405561-405795 | 468743-468844 | 496070-496120 | 509859-510009 | 550324-550331 |
| PDB 3I8Wb, Active Site | 396831-396862 | 405796-406030 | 468845-468946 | 496121-496171 | 510010-510160 | 550332-550339 |
| PDB 3KF0, Active Site | 396863-396894 | 406031-406265 | 468947-469048 | 496172-496222 | 510161-510311 | 550340-550347 |
| PDB 3KF0 with water, Active Site | 396895-396926 | 406266-406500 | 469049-469150 | 496223-496273 | 510312-510462 | 550348-550355 |
| PDB 3KFN, Active Site | 396927-396958 | 406501-406735 | 469151-469252 | 496274-496324 | 510463-510613 | 550356-550363 |
| PDB 3KFN with water, Active Site | 396959-396990 | 406736-406970 | 469253-469354 | 496325-496375 | 510614-510764 | 550364-550371 |
| PDB 3KFP, Active Site | 396991-397022 | 406971-407205 | 469355-469456 | 496376-496426 | 510765-510915 | 550372-550379 |
| PDB 3KFP with water, Active Site | 397023-397054 | 407206-407440 | 469457-469558 | 496427-496477 | 510916-511066 | 550380-550387 |
| PDB 3KFR, Active Site | 397055-397086 | 407441-407675 | 469559-469660 | 496478-496528 | 511067-511217 | 550388-550395 |
| PDB 3KFR with water, Active Site | 397087-397118 | 407676-407910 | 469661-469762 | 496529-496579 | 511218-511368 | 550396-550403 |
| PDB 3KFS, Active Site | 397119-397150 | 407911-408145 | 469763-469864 | 496580-496630 | 511369-511519 | 550404-550411 |
| PDB 3KFS with water, Active Site | 397151-397182 | 408146-408380 | 469865-469966 | 496631-496681 | 511520-511670 | 550412-550419 |
| PDB 3T11a, Active Site | 397183-397214 | 408381-408615 | 469967-470068 | 496682-496732 | 511671-511821 | 550420-550427 |
| PDB 3T11a with chloride, Active Site | 397215-397246 | 408616-408850 | 470069-470170 | 496733-496783 | 511822-511972 | 550428-550435 |
| PDB 3T11a with water, Active Site | 397247-397278 | 408851-409085 | 470171-470272 | 496784-496834 | 511973-512123 | 550436-550443 |
| PDB 3T11a with chloride and water, Active Site | 397279-397310 | 409086-409320 | 470273-470374 | 496835-496885 | 512124-512274 | 550444-550451 |
| PDB 3T11b, Active Site | 397311-397342 | 409321-409555 | 470375-470476 | 496886-496936 | 512275-512425 | 550452-550459 |
| PDB 3T11b with chloride, Active Site | 397343-397374 | 409556-409790 | 470477-470578 | 496937-496987 | 512426-512576 | 550460-550467 |
| PDB 3T11b with water, Active Site | 397375-397406 | 409791-410025 | 470579-470680 | 496988-497038 | 512577-512727 | 550468-550475 |
| PDB 3T11b with chloride and water, Active Site | 397407-397438 | 410026-410260 | 470681-470782 | 497039-497089 | 512728-512878 | 550476-550483 |
| PDB 4DQG, Active Site | 397439-397470 | 410261-410495 | 470783-470884 | 497090-497140 | 512879-513029 | 550484-550491 |
| PDB 4DQG with water, Active Site | 397471-397502 | 410496-410730 | 470885-470986 | 497141-497191 | 513030-513180 | 550492-550499 |
| PDB 4E43a, Active Site | 397503-397534 | 410731-410965 | 470987-471088 | 497192-497242 | 513181-513331 | 550500-550507 |
| PDB 4E43a with water, Active Site | 397535-397566 | 410966-411200 | 471089-471190 | 497243-497293 | 513332-513482 | 550508-550515 |
| PDB 4E43b, Active Site | 397567-397598 | 411201-411435 | 471191-471292 | 497294-497344 | 513483-513633 | 550516-550523 |
| PDB 4E43b with water, Active Site | 397599-397630 | 411436-411670 | 471293-471394 | 497345-497395 | 513634-513784 | 550524-550531 |
| COMPLETION | 13% | 67% | 99.7% | 100% | 99.9% | 95.8% |
Experiments 109 to 114 (AutoDock), 17% completed
Experiments 109 through 114 target the LEDGF site of HIV integrase. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M and Maybridge). These experiments will help distinguish better sites of among a group of receptor structures even within the same structure. To help read structures in the table below, the "a" and "b" after the 4-character PDB ID designate those receptor structures with alternate residue positions, while the "a" and "b" with the sites (FBP, LEDGF, Y3) designate different sites for the same symmetric structure of HIV IN.
| AD Exp. 109 | AD Exp. 110 | AD Exp. 111 | AD Exp. 112 | AD Exp. 113 | AD Exp. 114 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 4GVMa, LEDGFa Site | 374019-374335 | 381371-383715 | 377315-378328 | 375287-375793 | 390751-392254 | 373711-373787 |
| PDB 4GVMb, LEDGFa Site | 374336-374652 | 383716-386060 | 378329-379342 | 375794-376300 | 392255-393758 | 373788-373864 |
| PDB 4GW6a, LEDGFa Site | 374653-374969 | 386061-388405 | 379343-380356 | 376301-376807 | 393759-395262 | 373865-373941 |
| PDB 4GW6b, LEDGFa Site | 374970-375286 | 388406-390750 | 380357-381370 | 376808-377314 | 395263-396766 | 373942-374018 |
| COMPLETION | 19% | 14% | 0.05% | 46% | 24% | 0% |
Experiments 103 to 108 (AD Vina), 55% completed
Experiments 103 through 108 target allosteric sites of HIV integrase (FBP, LEDGF, and Y3 (under the 140s residues loop) sites). These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M) and Maybridge with AD Vina against various (receptor) structures from the PDB. These experiments complement Experiments 47 - 51, 91 - 96; however, these experiments will help distinguish better sites of among a group of receptor structures even within the same structure. For example, the HIV receptor structure containing the allosteric sites actually contains 2 sites for each of FBP, LEDGF, and Y3 pockets due to the symmetric nature of the HIV IN catalytic core domain (CCD). To help read structures in the table below, the "a" and "b" after the 4-character PDB ID designate those receptor structures with alternate residue positions, while the "a" and "b" with the sites (FBP, LEDGF, Y3) designate different sites for the same symmetric structure of HIV IN.
| AD Exp. 103 AD Vina Exp. 55 | AD Exp. 104 AD Vina Exp. 56 | AD Exp. 105 AD Vina Exp. 57 | AD Exp. 106 AD Vina Exp. 58 | AD Exp. 107 AD Vina Exp. 59 | AD Exp. 108 AD Vina Exp. 60 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 3AV9a, FBPa Site | 281071-281102 | 286191-286425 | 323791-323892 | 340111-340161 | 348271-348421 | 372431-372438 |
| PDB 3AV9a, FBPb Site | 281103-281134 | 286426-286660 | 323893-323994 | 340162-340212 | 348422-348572 | 372439-372446 |
| PDB 3AV9a, LEDGFa Site | 281135-281166 | 286661-286895 | 323995-324096 | 340213-340263 | 348573-348723 | 372447-372454 |
| PDB 3AV9a, LEDGFb Site | 281167-281198 | 286896-287130 | 324097-324198 | 340264-340314 | 348724-348874 | 372455-372462 |
| PDB 3AV9a, Y3a Site | 281199-281230 | 287131-287365 | 324199-324300 | 340315-340365 | 348875-349025 | 372463-372470 |
| PDB 3AV9a, Y3b Site | 281231-281262 | 287366-287600 | 324301-324402 | 340366-340416 | 349026-349176 | 372471-372478 |
| PDB 3AV9b, FBPa Site | 281263-281294 | 287601-287835 | 324403-324504 | 340417-340467 | 349177-349327 | 372479-372486 |
| PDB 3AV9b, FBPb Site | 281295-281326 | 287836-288070 | 324505-324606 | 340468-340518 | 349328-349478 | 372487-372494 |
| PDB 3AV9b, LEDGFa Site | 281327-281358 | 288071-288305 | 324607-324708 | 340519-340569 | 349479-349629 | 372495-372502 |
| PDB 3AV9b, LEDGFb Site | 281359-281390 | 288306-288540 | 324709-324810 | 340570-340620 | 349630-349780 | 372503-372510 |
| PDB 3AV9b, Y3a Site | 281391-281422 | 288541-288775 | 324811-324912 | 340621-340671 | 349781-349931 | 372511-372518 |
| PDB 3AV9b, Y3b Site | 281423-281454 | 288776-289010 | 324913-325014 | 340672-340722 | 349932-350082 | 372519-372526 |
| PDB 3AVAa, FBPa Site | 281455-281486 | 289011-289245 | 325015-325116 | 340723-340773 | 350083-350233 | 372527-372534 |
| PDB 3AVAa, FBPb Site | 281487-281518 | 289246-289480 | 325117-325218 | 340774-340824 | 350234-350384 | 372535-372542 |
| PDB 3AVAa, LEDGFa Site | 281519-281550 | 289481-289715 | 325219-325320 | 340825-340875 | 350385-350535 | 372543-372550 |
| PDB 3AVAa, LEDGFb Site | 281551-281582 | 289716-289950 | 325321-325422 | 340876-340926 | 350536-350686 | 372551-372558 |
| PDB 3AVAa, Y3a Site | 281583-281614 | 289951-290185 | 325423-325524 | 340927-340977 | 350687-350837 | 372559-372566 |
| PDB 3AVAa, Y3b Site | 281615-281646 | 290186-290420 | 325525-325626 | 340978-341028 | 350838-350988 | 372567-372574 |
| PDB 3AVAb, FBPa Site | 281647-281678 | 290421-290655 | 325627-325728 | 341029-341079 | 350989-351139 | 372575-372582 |
| PDB 3AVAb, FBPb Site | 281679-281710 | 290656-290890 | 325729-325830 | 341080-341130 | 351140-351290 | 372583-372590 |
| PDB 3AVAb, LEDGFa Site | 281711-281742 | 290891-291125 | 325831-325932 | 341131-341181 | 351291-351441 | 372591-372598 |
| PDB 3AVAb, LEDGFb Site | 281743-281774 | 291126-291360 | 325933-326034 | 341182-341232 | 351442-351592 | 372599-372606 |
| PDB 3AVAb, Y3a Site | 281775-281806 | 291361-291595 | 326035-326136 | 341233-341283 | 351593-351743 | 372607-372614 |
| PDB 3AVAb, Y3b Site | 281807-281838 | 291596-291830 | 326137-326238 | 341284-341334 | 351744-351894 | 372615-372622 |
| PDB 3AVCa, FBPa Site | 281839-281870 | 291831-292065 | 326239-326340 | 341335-341385 | 351895-352045 | 372623-372630 |
| PDB 3AVCa, FBPb Site | 281871-281902 | 292066-292300 | 326341-326442 | 341386-341436 | 352046-352196 | 372631-372638 |
| PDB 3AVCa, LEDGFa Site | 281903-281934 | 292301-292535 | 326443-326544 | 341437-341487 | 352197-352347 | 372639-372646 |
| PDB 3AVCa, LEDGFb Site | 281935-281966 | 292536-292770 | 326545-326646 | 341488-341538 | 352348-352498 | 372647-372654 |
| PDB 3AVCa, Y3a Site | 281967-281998 | 292771-293005 | 326647-326748 | 341539-341589 | 352499-352649 | 372655-372662 |
| PDB 3AVCa, Y3b Site | 281999-282030 | 293006-293240 | 326749-326850 | 341590-341640 | 352650-352800 | 372663-372670 |
| PDB 3AVCb, FBPa Site | 282031-282062 | 293241-293475 | 326851-326952 | 341641-341691 | 352801-352951 | 372671-372678 |
| PDB 3AVCb, FBPb Site | 282063-282094 | 293476-293710 | 326953-327054 | 341692-341742 | 352952-353102 | 372679-372686 |
| PDB 3AVCb, LEDGFa Site | 282095-282126 | 293711-293945 | 327055-327156 | 341743-341793 | 353103-353253 | 372687-372694 |
| PDB 3AVCb, LEDGFb Site | 282127-282158 | 293946-294180 | 327157-327258 | 341794-341844 | 353254-353404 | 372695-372702 |
| PDB 3AVCb, Y3a Site | 282159-282190 | 294181-294415 | 327259-327360 | 341845-341895 | 353405-353555 | 372703-372710 |
| PDB 3AVCb, Y3b Site | 282191-282222 | 294416-294650 | 327361-327462 | 341896-341946 | 353556-353706 | 372711-372718 |
| PDB 3AVFa, FBPa Site | 282223-282254 | 294651-294885 | 327463-327564 | 341947-341997 | 353707-353857 | 372719-372726 |
| PDB 3AVFa, FBPb Site | 282255-282286 | 294886-295120 | 327565-327666 | 341998-342048 | 353858-354008 | 372727-372734 |
| PDB 3AVFa, LEDGFa Site | 282287-282318 | 295121-295355 | 327667-327768 | 342049-342099 | 354009-354159 | 372735-372742 |
| PDB 3AVFa, LEDGFb Site | 282319-282350 | 295356-295590 | 327769-327870 | 342100-342150 | 354160-354310 | 372743-372750 |
| PDB 3AVFa, Y3a Site | 282351-282382 | 295591-295825 | 327871-327972 | 342151-342201 | 354311-354461 | 372751-372758 |
| PDB 3AVFa, Y3b Site | 282383-282414 | 295826-296060 | 327973-328074 | 342202-342252 | 354462-354612 | 372759-372766 |
| PDB 3AVFb, FBPa Site | 282415-282446 | 296061-296295 | 328075-328176 | 342253-342303 | 354613-354763 | 372767-372774 |
| PDB 3AVFb, FBPb Site | 282447-282478 | 296296-296530 | 328177-328278 | 342304-342354 | 354764-354914 | 372775-372782 |
| PDB 3AVFb, LEDGFa Site | 282479-282510 | 296531-296765 | 328279-328380 | 342355-342405 | 354915-355065 | 372783-372790 |
| PDB 3AVFb, LEDGFb Site | 282511-282542 | 296766-297000 | 328381-328482 | 342406-342456 | 355066-355216 | 372791-372798 |
| PDB 3AVFb, Y3a Site | 282543-282574 | 297001-297235 | 328483-328584 | 342457-342507 | 355217-355367 | 372799-372806 |
| PDB 3AVFb, Y3b Site | 282575-282606 | 297236-297470 | 328585-328686 | 342508-342558 | 355368-355518 | 372807-372814 |
| PDB 3AVGa, FBPa Site | 282607-282638 | 297471-297705 | 328687-328788 | 342559-342609 | 355519-355669 | 372815-372822 |
| PDB 3AVGa, FBPb Site | 282639-282670 | 297706-297940 | 328789-328890 | 342610-342660 | 355670-355820 | 372823-372830 |
| PDB 3AVGa, LEDGFa Site | 282671-282702 | 297941-298175 | 328891-328992 | 342661-342711 | 355821-355971 | 372831-372838 |
| PDB 3AVGa, LEDGFb Site | 282703-282734 | 298176-298410 | 328993-329094 | 342712-342762 | 355972-356122 | 372839-372846 |
| PDB 3AVGa, Y3a Site | 282735-282766 | 298411-298645 | 329095-329196 | 342763-342813 | 356123-356273 | 372847-372854 |
| PDB 3AVGa, Y3b Site | 282767-282798 | 298646-298880 | 329197-329298 | 342814-342864 | 356274-356424 | 372855-372862 |
| PDB 3AVGb, FBPa Site | 282799-282830 | 298881-299115 | 329299-329400 | 342865-342915 | 356425-356575 | 372863-372870 |
| PDB 3AVGb, FBPb Site | 282831-282862 | 299116-299350 | 329401-329502 | 342916-342966 | 356576-356726 | 372871-372878 |
| PDB 3AVGb, LEDGFa Site | 282863-282894 | 299351-299585 | 329503-329604 | 342967-343017 | 356727-356877 | 372879-372886 |
| PDB 3AVGb, LEDGFb Site | 282895-282926 | 299586-299820 | 329605-329706 | 343018-343068 | 356878-357028 | 372887-372894 |
| PDB 3AVGb, Y3a Site | 282927-282958 | 299821-300055 | 329707-329808 | 343069-343119 | 357029-357179 | 372895-372902 |
| PDB 3AVGb, Y3b Site | 282959-282990 | 300056-300290 | 329809-329910 | 343120-343170 | 357180-357330 | 372903-372910 |
| PDB 3AVHa, FBPa Site | 282991-283022 | 300291-300525 | 329911-330012 | 343171-343221 | 357331-357481 | 372911-372918 |
| PDB 3AVHa, FBPb Site | 283023-283054 | 300526-300760 | 330013-330114 | 343222-343272 | 357482-357632 | 372919-372926 |
| PDB 3AVHa, LEDGFa Site | 283055-283086 | 300761-300995 | 330115-330216 | 343273-343323 | 357633-357783 | 372927-372934 |
| PDB 3AVHa, LEDGFb Site | 283087-283118 | 300996-301230 | 330217-330318 | 343324-343374 | 357784-357934 | 372935-372942 |
| PDB 3AVHa, Y3a Site | 283119-283150 | 301231-301465 | 330319-330420 | 343375-343425 | 357935-358085 | 372943-372950 |
| PDB 3AVHa, Y3b Site | 283151-283182 | 301466-301700 | 330421-330522 | 343426-343476 | 358086-358236 | 372951-372958 |
| PDB 3AVHb, FBPa Site | 283183-283214 | 301701-301935 | 330523-330624 | 343477-343527 | 358237-358387 | 372959-372966 |
| PDB 3AVHb, FBPb Site | 283215-283246 | 301936-302170 | 330625-330726 | 343528-343578 | 358388-358538 | 372967-372974 |
| PDB 3AVHb, LEDGFa Site | 283247-283278 | 302171-302405 | 330727-330828 | 343579-343629 | 358539-358689 | 372975-372982 |
| PDB 3AVHb, LEDGFb Site | 283279-283310 | 302406-302640 | 330829-330930 | 343630-343680 | 358690-358840 | 372983-372990 |
| PDB 3AVHb, Y3a Site | 283311-283342 | 302641-302875 | 330931-331032 | 343681-343731 | 358841-358991 | 372991-372998 |
| PDB 3AVHb, Y3b Site | 283343-283374 | 302876-303110 | 331033-331134 | 343732-343782 | 358992-359142 | 372999-373006 |
| PDB 3AVIa, FBPa Site | 283375-283406 | 303111-303345 | 331135-331236 | 343783-343833 | 359143-359293 | 373007-373014 |
| PDB 3AVIa, FBPb Site | 283407-283438 | 303346-303580 | 331237-331338 | 343834-343884 | 359294-359444 | 373015-373022 |
| PDB 3AVIa, LEDGFa Site | 283439-283470 | 303581-303815 | 331339-331440 | 343885-343935 | 359445-359595 | 373023-373030 |
| PDB 3AVIa, LEDGFb Site | 283471-283502 | 303816-304050 | 331441-331542 | 343936-343986 | 359596-359746 | 373031-373038 |
| PDB 3AVIa, Y3a Site | 283503-283534 | 304051-304285 | 331543-331644 | 343987-344037 | 359747-359897 | 373039-373046 |
| PDB 3AVIa, Y3b Site | 283535-283566 | 304286-304520 | 331645-331746 | 344038-344088 | 359898-360048 | 373047-373054 |
| PDB 3AVIb, FBPa Site | 283567-283598 | 304521-304755 | 331747-331848 | 344089-344139 | 360049-360199 | 373055-373062 |
| PDB 3AVIb, FBPb Site | 283599-283630 | 304756-304990 | 331849-331950 | 344140-344190 | 360200-360350 | 373063-373070 |
| PDB 3AVIb, LEDGFa Site | 283631-283662 | 304991-305225 | 331951-332052 | 344191-344241 | 360351-360501 | 373071-373078 |
| PDB 3AVIb, LEDGFb Site | 283663-283694 | 305226-305460 | 332053-332154 | 344242-344292 | 360502-360652 | 373079-373086 |
| PDB 3AVIb, Y3a Site | 283695-283726 | 305461-305695 | 332155-332256 | 344293-344343 | 360653-360803 | 373087-373094 |
| PDB 3AVIb, Y3b Site | 283727-283758 | 305696-305930 | 332257-332358 | 344344-344394 | 360804-360954 | 373095-373102 |
| PDB 3AVJa, FBPa Site | 283759-283790 | 305931-306165 | 332359-332460 | 344395-344445 | 360955-361105 | 373103-373110 |
| PDB 3AVJa, FBPb Site | 283791-283822 | 306166-306400 | 332461-332562 | 344446-344496 | 361106-361256 | 373111-373118 |
| PDB 3AVJa, LEDGFa Site | 283823-283854 | 306401-306635 | 332563-332664 | 344497-344547 | 361257-361407 | 373119-373126 |
| PDB 3AVJa, LEDGFb Site | 283855-283886 | 306636-306870 | 332665-332766 | 344548-344598 | 361408-361558 | 373127-373134 |
| PDB 3AVJa, Y3a Site | 283887-283918 | 306871-307105 | 332767-332868 | 344599-344649 | 361559-361709 | 373135-373142 |
| PDB 3AVJa, Y3b Site | 283919-283950 | 307106-307340 | 332869-332970 | 344650-344700 | 361710-361860 | 373143-373150 |
| PDB 3AVJb, FBPa Site | 283951-283982 | 307341-307575 | 332971-333072 | 344701-344751 | 361861-362011 | 373151-373158 |
| PDB 3AVJb, FBPb Site | 283983-284014 | 307576-307810 | 333073-333174 | 344752-344802 | 362012-362162 | 373159-373166 |
| PDB 3AVJb, LEDGFa Site | 284015-284046 | 307811-308045 | 333175-333276 | 344803-344853 | 362163-362313 | 373167-373174 |
| PDB 3AVJb, LEDGFb Site | 284047-284078 | 308046-308280 | 333277-333378 | 344854-344904 | 362314-362464 | 373175-373182 |
| PDB 3AVJb, Y3a Site | 284079-284110 | 308281-308515 | 333379-333480 | 344905-344955 | 362465-362615 | 373183-373190 |
| PDB 3AVJb, Y3b Site | 284111-284142 | 308516-308750 | 333481-333582 | 344956-345006 | 362616-362766 | 373191-373198 |
| PDB 3AVKa, FBPa Site | 284143-284174 | 308751-308985 | 333583-333684 | 345007-345057 | 362767-362917 | 373199-373206 |
| PDB 3AVKa, FBPb Site | 284175-284206 | 308986-309220 | 333685-333786 | 345058-345108 | 362918-363068 | 373207-373214 |
| PDB 3AVKa, LEDGFa Site | 284207-284238 | 309221-309455 | 333787-333888 | 345109-345159 | 363069-363219 | 373215-373222 |
| PDB 3AVKa, LEDGFb Site | 284239-284270 | 309456-309690 | 333889-333990 | 345160-345210 | 363220-363370 | 373223-373230 |
| PDB 3AVKa, Y3a Site | 284271-284302 | 309691-309925 | 333991-334092 | 345211-345261 | 363371-363521 | 373231-373238 |
| PDB 3AVKa, Y3b Site | 284303-284334 | 309926-310160 | 334093-334194 | 345262-345312 | 363522-363672 | 373239-373246 |
| PDB 3AVKb, FBPa Site | 284335-284366 | 310161-310395 | 334195-334296 | 345313-345363 | 363673-363823 | 373247-373254 |
| PDB 3AVKb, FBPb Site | 284367-284398 | 310396-310630 | 334297-334398 | 345364-345414 | 363824-363974 | 373255-373262 |
| PDB 3AVKb, LEDGFa Site | 284399-284430 | 310631-310865 | 334399-334500 | 345415-345465 | 363975-364125 | 373263-373270 |
| PDB 3AVKb, LEDGFb Site | 284431-284462 | 310866-311100 | 334501-334602 | 345466-345516 | 364126-364276 | 373271-373278 |
| PDB 3AVKb, Y3a Site | 284463-284494 | 311101-311335 | 334603-334704 | 345517-345567 | 364277-364427 | 373279-373286 |
| PDB 3AVKb, Y3b Site | 284495-284526 | 311336-311570 | 334705-334806 | 345568-345618 | 364428-364578 | 373287-373294 |
| PDB 3AVLa, FBPa Site | 284527-284558 | 311571-311805 | 334807-334908 | 345619-345669 | 364579-364729 | 373295-373302 |
| PDB 3AVLa, FBPb Site | 284559-284590 | 311806-312040 | 334909-335010 | 345670-345720 | 364730-364880 | 373303-373310 |
| PDB 3AVLa, LEDGFa Site | 284591-284622 | 312041-312275 | 335011-335112 | 345721-345771 | 364881-365031 | 373311-373318 |
| PDB 3AVLa, LEDGFb Site | 284623-284654 | 312276-312510 | 335113-335214 | 345772-345822 | 365032-365182 | 373319-373326 |
| PDB 3AVLa, Y3a Site | 284655-284686 | 312511-312745 | 335215-335316 | 345823-345873 | 365183-365333 | 373327-373334 |
| PDB 3AVLa, Y3b Site | 284687-284718 | 312746-312980 | 335317-335418 | 345874-345924 | 365334-365484 | 373335-373342 |
| PDB 3AVLb, FBPa Site | 284719-284750 | 312981-313215 | 335419-335520 | 345925-345975 | 365485-365635 | 373343-373350 |
| PDB 3AVLb, FBPb Site | 284751-284782 | 313216-313450 | 335521-335622 | 345976-346026 | 365636-365786 | 373351-373358 |
| PDB 3AVLb, LEDGFa Site | 284783-284814 | 313451-313685 | 335623-335724 | 346027-346077 | 365787-365937 | 373359-373366 |
| PDB 3AVLb, LEDGFb Site | 284815-284846 | 313686-313920 | 335725-335826 | 346078-346128 | 365938-366088 | 373367-373374 |
| PDB 3AVLb, Y3a Site | 284847-284878 | 313921-314155 | 335827-335928 | 346129-346179 | 366089-366239 | 373375-373382 |
| PDB 3AVLb, Y3b Site | 284879-284910 | 314156-314390 | 335929-336030 | 346180-346230 | 366240-366390 | 373383-373390 |
| PDB 3AVMa, FBPa Site | 284911-284942 | 314391-314625 | 336031-336132 | 346231-346281 | 366391-366541 | 373391-373398 |
| PDB 3AVMa, FBPb Site | 284943-284974 | 314626-314860 | 336133-336234 | 346282-346332 | 366542-366692 | 373399-373406 |
| PDB 3AVMa, LEDGFa Site | 284975-285006 | 314861-315095 | 336235-336336 | 346333-346383 | 366693-366843 | 373407-373414 |
| PDB 3AVMa, LEDGFb Site | 285007-285038 | 315096-315330 | 336337-336438 | 346384-346434 | 366844-366994 | 373415-373422 |
| PDB 3AVMa, Y3a Site | 285039-285070 | 315331-315565 | 336439-336540 | 346435-346485 | 366995-367145 | 373423-373430 |
| PDB 3AVMa, Y3b Site | 285071-285102 | 315566-315800 | 336541-336642 | 346486-346536 | 367146-367296 | 373431-373438 |
| PDB 3AVMb, FBPa Site | 285103-285134 | 315801-316035 | 336643-336744 | 346537-346587 | 367297-367447 | 373439-373446 |
| PDB 3AVMb, FBPb Site | 285135-285166 | 316036-316270 | 336745-336846 | 346588-346638 | 367448-367598 | 373447-373454 |
| PDB 3AVMb, LEDGFa Site | 285167-285198 | 316271-316505 | 336847-336948 | 346639-346689 | 367599-367749 | 373455-373462 |
| PDB 3AVMb, LEDGFb Site | 285199-285230 | 316506-316740 | 336949-337050 | 346690-346740 | 367750-367900 | 373463-373470 |
| PDB 3AVMb, Y3a Site | 285231-285262 | 316741-316975 | 337051-337152 | 346741-346791 | 367901-368051 | 373471-373478 |
| PDB 3AVMb, Y3b Site | 285263-285294 | 316976-317210 | 337153-337254 | 346792-346842 | 368052-368202 | 373479-373486 |
| PDB 3AVNa, FBPa Site | 285295-285326 | 317211-317445 | 337255-337356 | 346843-346893 | 368203-368353 | 373487-373494 |
| PDB 3AVNa, FBPb Site | 285327-285358 | 317446-317680 | 337357-337458 | 346894-346944 | 368354-368504 | 373495-373502 |
| PDB 3AVNa, LEDGFa Site | 285359-285390 | 317681-317915 | 337459-337560 | 346945-346995 | 368505-368655 | 373503-373510 |
| PDB 3AVNa, LEDGFb Site | 285391-285422 | 317916-318150 | 337561-337662 | 346996-347046 | 368656-368806 | 373511-373518 |
| PDB 3AVNa, Y3a Site | 285423-285454 | 318151-318385 | 337663-337764 | 347047-347097 | 368807-368957 | 373519-373526 |
| PDB 3AVNa, Y3b Site | 285455-285486 | 318386-318620 | 337765-337866 | 347098-347148 | 368958-369108 | 373527-373534 |
| PDB 3AVNb, FBPa Site | 285487-285518 | 318621-318855 | 337867-337968 | 347149-347199 | 369109-369259 | 373535-373542 |
| PDB 3AVNb, FBPb Site | 285519-285550 | 318856-319090 | 337969-338070 | 347200-347250 | 369260-369410 | 373543-373550 |
| PDB 3AVNb, LEDGFa Site | 285551-285582 | 319091-319325 | 338071-338172 | 347251-347301 | 369411-369561 | 373551-373558 |
| PDB 3AVNb, LEDGFb Site | 285583-285614 | 319326-319560 | 338173-338274 | 347302-347352 | 369562-369712 | 373559-373566 |
| PDB 3AVNb, Y3a Site | 285615-285646 | 319561-319795 | 338275-338376 | 347353-347403 | 369713-369863 | 373567-373574 |
| PDB 3AVNb, Y3b Site | 285647-285678 | 319796-320030 | 338377-338478 | 347404-347454 | 369864-370014 | 373575-373582 |
| PDB 4GVMa, FBPa Site | 285679-285710 | 320031-320265 | 338479-338580 | 347455-347505 | 370015-370165 | 373583-373590 |
| PDB 4GVMa, FBPb Site | 285711-285742 | 320266-320500 | 338581-338682 | 347506-347556 | 370166-370316 | 373591-373598 |
| PDB 4GVMa, LEDGFa Site | 285743-285774 | 320501-320735 | 338683-338784 | 347557-347607 | 370317-370467 | 373599-373606 |
| PDB 4GVMa, LEDGFb Site | 285775-285806 | 320736-320970 | 338785-338886 | 347608-347658 | 370468-370618 | 373607-373614 |
| PDB 4GVMb, FBPa Site | 285807-285838 | 320971-321205 | 338887-338988 | 347659-347709 | 370619-370769 | 373615-373622 |
| PDB 4GVMb, FBPb Site | 285839-285870 | 321206-321440 | 338989-339090 | 347710-347760 | 370770-370920 | 373623-373630 |
| PDB 4GVMb, LEDGFa Site | 285871-285902 | 321441-321675 | 339091-339192 | 347761-347811 | 370921-371071 | 373631-373638 |
| PDB 4GVMb, LEDGFb Site | 285903-285934 | 321676-321910 | 339193-339294 | 347812-347862 | 371072-371222 | 373639-373646 |
| PDB 4GW6a, FBPa Site | 285935-285966 | 321911-322145 | 339295-339396 | 347863-347913 | 371223-371373 | 373647-373654 |
| PDB 4GW6a, FBPb Site | 285967-285998 | 322146-322380 | 339397-339498 | 347914-347964 | 371374-371524 | 373655-373662 |
| PDB 4GW6a, LEDGFa Site | 285999-286030 | 322381-322615 | 339499-339600 | 347965-348015 | 371525-371675 | 373663-373670 |
| PDB 4GW6a, LEDGFb Site | 286031-286062 | 322616-322850 | 339601-339702 | 348016-348066 | 371676-371826 | 373671-373678 |
| PDB 4GW6b, FBPa Site | 286063-286094 | 322851-323085 | 339703-339804 | 348067-348117 | 371827-371977 | 373679-373686 |
| PDB 4GW6b, FBPb Site | 286095-286126 | 323086-323320 | 339805-339906 | 348118-348168 | 371978-372128 | 373687-373694 |
| PDB 4GW6b, LEDGFa Site | 286127-286158 | 323321-323555 | 339907-340008 | 348169-348219 | 372129-372279 | 373695-373702 |
| PDB 4GW6b, LEDGFb Site | 286159-286190 | 323556-323790 | 340009-340110 | 348220-348270 | 372280-372430 | 373703-373710 |
| COMPLETION | 83% | 90% | 34% | 100% | 26% | 0% |
Status 72-102
Experiments 97 to 102 (AD Vina), 13% completed
Experiments 97 through 102 target allosteric sites of HIV integrase (FBP, LEDGF, and Y3 (under the 140s residues loop) sites) using the flexible residue feature in AutoDock Vina. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M) and Maybridge with AD Vina against various structures from the PDB. These experiments complement Experiments 47 - 51, 91 - 96; and the resulting data will help discriminate receptor structures when many are available for a virtual screening as well as evaluate flexible docking with AutoDock Vina.
| AD Exp. 97 AD Vina Exp. 49 | AD Exp. 98 AD Vina Exp. 50 | AD Exp. 99 AD Vina Exp. 51 | AD Exp. 100 AD Vina Exp. 52 | AD Exp. 101 AD Vina Exp. 53 | AD Exp. 102 AD Vina Exp. 54 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 3AO1, FBPa Site | 197695-197726 | 202303-202537 | 236143-236244 | 250831-250881 | 258175-258325 | 279919-279926 |
| PDB 3AO1, FBPb Site | 197727-197758 | 202538-202772 | 236245-236346 | 250882-250932 | 258326-258476 | 279927-279934 |
| PDB 3AO1, LEDGFa Site | 197759-197790 | 202773-203007 | 236347-236448 | 250933-250983 | 258477-258627 | 279935-279942 |
| PDB 3AO1, LEDGFb Site | 197791-197822 | 203008-203242 | 236449-236550 | 250984-251034 | 258628-258778 | 279943-279950 |
| PDB 3AO2, FBPa Site | 197823-197854 | 203243-203477 | 236551-236652 | 251035-251085 | 258779-258929 | 279951-279958 |
| PDB 3AO2, FBPb Site | 197855-197886 | 203478-203712 | 236653-236754 | 251086-251136 | 258930-259080 | 279959-279966 |
| PDB 3AO2, LEDGFa Site | 197887-197918 | 203713-203947 | 236755-236856 | 251137-251187 | 259081-259231 | 279967-279974 |
| PDB 3AO2, LEDGFb Site | 197919-197950 | 203948-204182 | 236857-236958 | 251188-251238 | 259232-259382 | 279975-279982 |
| PDB 3NF6, FBPa Site | 197951-197982 | 204183-204417 | 236959-237060 | 251239-251289 | 259383-259533 | 279983-279990 |
| PDB 3NF6, FBPb Site | 197983-198014 | 204418-204652 | 237061-237162 | 251290-251340 | 259534-259684 | 279991-279998 |
| PDB 3NF6, LEDGFa Site | 198015-198046 | 204653-204887 | 237163-237264 | 251341-251391 | 259685-259835 | 279999-280006 |
| PDB 3NF6, LEDGFb Site | 198047-198078 | 204888-205122 | 237265-237366 | 251392-251442 | 259836-259986 | 280007-280014 |
| PDB 3NF6, Y3a Site | 198079-198110 | 205123-205357 | 237367-237468 | 251443-251493 | 259987-260137 | 280015-280022 |
| PDB 3NF6, Y3b Site | 198111-198142 | 205358-205592 | 237469-237570 | 251494-251544 | 260138-260288 | 280023-280030 |
| PDB 3NF6, FBPa Site | 198143-198174 | 205593-205827 | 237571-237672 | 251545-251595 | 260289-260439 | 280031-280038 |
| PDB 3NF6, FBPb Site | 198175-198206 | 205828-206062 | 237673-237774 | 251596-251646 | 260440-260590 | 280039-280046 |
| PDB 3NF6, LEDGFa Site | 198207-198238 | 206063-206297 | 237775-237876 | 251647-251697 | 260591-260741 | 280047-280054 |
| PDB 3NF6, LEDGFb Site | 198239-198270 | 206298-206532 | 237877-237978 | 251698-251748 | 260742-260892 | 280055-280062 |
| PDB 3NF6, Y3a Site | 198271-198302 | 206533-206767 | 237979-238080 | 251749-251799 | 260893-261043 | 280063-280070 |
| PDB 3NF6, Y3b Site | 198303-198334 | 206768-207002 | 238081-238182 | 251800-251850 | 261044-261194 | 280071-280078 |
| PDB 3NF7, FBPa Site | 198335-198366 | 207003-207237 | 238183-238284 | 251851-251901 | 261195-261345 | 280079-280086 |
| PDB 3NF7, FBPb Site | 198367-198398 | 207238-207472 | 238285-238386 | 251902-251952 | 261346-261496 | 280087-280094 |
| PDB 3NF7, LEDGFa Site | 198399-198430 | 207473-207707 | 238387-238488 | 251953-252003 | 261497-261647 | 280095-280102 |
| PDB 3NF7, LEDGFb Site | 198431-198462 | 207708-207942 | 238489-238590 | 252004-252054 | 261648-261798 | 280103-280110 |
| PDB 3NF7, Y3a Site | 198463-198494 | 207943-208177 | 238591-238692 | 252055-252105 | 261799-261949 | 280111-280118 |
| PDB 3NF7, Y3b Site | 198495-198526 | 208178-208412 | 238693-238794 | 252106-252156 | 261950-262100 | 280119-280126 |
| PDB 3NF7, FBPa Site | 198527-198558 | 208413-208647 | 238795-238896 | 252157-252207 | 262101-262251 | 280127-280134 |
| PDB 3NF7, FBPb Site | 198559-198590 | 208648-208882 | 238897-238998 | 252208-252258 | 262252-262402 | 280135-280142 |
| PDB 3NF7, LEDGFa Site | 198591-198622 | 208883-209117 | 238999-239100 | 252259-252309 | 262403-262553 | 280143-280150 |
| PDB 3NF7, LEDGFb Site | 198623-198654 | 209118-209352 | 239101-239202 | 252310-252360 | 262554-262704 | 280151-280158 |
| PDB 3NF7, Y3a Site | 198655-198686 | 209353-209587 | 239203-239304 | 252361-252411 | 262705-262855 | 280159-280166 |
| PDB 3NF7, Y3b Site | 198687-198718 | 209588-209822 | 239305-239406 | 252412-252462 | 262856-263006 | 280167-280174 |
| PDB 3NF8, FBPa Site | 198719-198750 | 209823-210057 | 239407-239508 | 252463-252513 | 263007-263157 | 280175-280182 |
| PDB 3NF8, FBPb Site | 198751-198782 | 210058-210292 | 239509-239610 | 252514-252564 | 263158-263308 | 280183-280190 |
| PDB 3NF8, LEDGFa Site | 198783-198814 | 210293-210527 | 239611-239712 | 252565-252615 | 263309-263459 | 280191-280198 |
| PDB 3NF8, LEDGFb Site | 198815-198846 | 210528-210762 | 239713-239814 | 252616-252666 | 263460-263610 | 280199-280206 |
| PDB 3NF8, Y3a Site | 198847-198878 | 210763-210997 | 239815-239916 | 252667-252717 | 263611-263761 | 280207-280214 |
| PDB 3NF8, Y3b Site | 198879-198910 | 210998-211232 | 239917-240018 | 252718-252768 | 263762-263912 | 280215-280222 |
| PDB 3NF8, FBPa Site | 198911-198942 | 211233-211467 | 240019-240120 | 252769-252819 | 263913-264063 | 280223-280230 |
| PDB 3NF8, FBPb Site | 198943-198974 | 211468-211702 | 240121-240222 | 252820-252870 | 264064-264214 | 280231-280238 |
| PDB 3NF8, LEDGFa Site | 198975-199006 | 211703-211937 | 240223-240324 | 252871-252921 | 264215-264365 | 280239-280246 |
| PDB 3NF8, LEDGFb Site | 199007-199038 | 211938-212172 | 240325-240426 | 252922-252972 | 264366-264516 | 280247-280254 |
| PDB 3NF8, Y3a Site | 199039-199070 | 212173-212407 | 240427-240528 | 252973-253023 | 264517-264667 | 280255-280262 |
| PDB 3NF8, Y3b Site | 199071-199102 | 212408-212642 | 240529-240630 | 253024-253074 | 264668-264818 | 280263-280270 |
| PDB 3NF9, FBPa Site | 199103-199134 | 212643-212877 | 240631-240732 | 253075-253125 | 264819-264969 | 280271-280278 |
| PDB 3NF9, FBPb Site | 199135-199166 | 212878-213112 | 240733-240834 | 253126-253176 | 264970-265120 | 280279-280286 |
| PDB 3NF9, LEDGFa Site | 199167-199198 | 213113-213347 | 240835-240936 | 253177-253227 | 265121-265271 | 280287-280294 |
| PDB 3NF9, LEDGFb Site | 199199-199230 | 213348-213582 | 240937-241038 | 253228-253278 | 265272-265422 | 280295-280302 |
| PDB 3NF9, Y3a Site | 199231-199262 | 213583-213817 | 241039-241140 | 253279-253329 | 265423-265573 | 280303-280310 |
| PDB 3NF9, Y3b Site | 199263-199294 | 213818-214052 | 241141-241242 | 253330-253380 | 265574-265724 | 280311-280318 |
| PDB 3NF9, FBPa Site | 199295-199326 | 214053-214287 | 241243-241344 | 253381-253431 | 265725-265875 | 280319-280326 |
| PDB 3NF9, FBPb Site | 199327-199358 | 214288-214522 | 241345-241446 | 253432-253482 | 265876-266026 | 280327-280334 |
| PDB 3NF9, LEDGFa Site | 199359-199390 | 214523-214757 | 241447-241548 | 253483-253533 | 266027-266177 | 280335-280342 |
| PDB 3NF9, LEDGFb Site | 199391-199422 | 214758-214992 | 241549-241650 | 253534-253584 | 266178-266328 | 280343-280350 |
| PDB 3NF9, Y3a Site | 199423-199454 | 214993-215227 | 241651-241752 | 253585-253635 | 266329-266479 | 280351-280358 |
| PDB 3NF9, Y3b Site | 199455-199486 | 215228-215462 | 241753-241854 | 253636-253686 | 266480-266630 | 280359-280366 |
| PDB 3NFA, FBPa Site | 199487-199518 | 215463-215697 | 241855-241956 | 253687-253737 | 266631-266781 | 280367-280374 |
| PDB 3NFA, FBPb Site | 199519-199550 | 215698-215932 | 241957-242058 | 253738-253788 | 266782-266932 | 280375-280382 |
| PDB 3NFA, LEDGFa Site | 199551-199582 | 215933-216167 | 242059-242160 | 253789-253839 | 266933-267083 | 280383-280390 |
| PDB 3NFA, LEDGFb Site | 199583-199614 | 216168-216402 | 242161-242262 | 253840-253890 | 267084-267234 | 280391-280398 |
| PDB 3NFA, Y3a Site | 199615-199646 | 216403-216637 | 242263-242364 | 253891-253941 | 267235-267385 | 280399-280406 |
| PDB 3NFA, Y3b Site | 199647-199678 | 216638-216872 | 242365-242466 | 253942-253992 | 267386-267536 | 280407-280414 |
| PDB 3NFA, FBPa Site | 199679-199710 | 216873-217107 | 242467-242568 | 253993-254043 | 267537-267687 | 280415-280422 |
| PDB 3NFA, FBPb Site | 199711-199742 | 217108-217342 | 242569-242670 | 254044-254094 | 267688-267838 | 280423-280430 |
| PDB 3NFA, LEDGFa Site | 199743-199774 | 217343-217577 | 242671-242772 | 254095-254145 | 267839-267989 | 280431-280438 |
| PDB 3NFA, LEDGFb Site | 199775-199806 | 217578-217812 | 242773-242874 | 254146-254196 | 267990-268140 | 280439-280446 |
| PDB 3NFA, Y3a Site | 199807-199838 | 217813-218047 | 242875-242976 | 254197-254247 | 268141-268291 | 280447-280454 |
| PDB 3NFA, Y3b Site | 199839-199870 | 218048-218282 | 242977-243078 | 254248-254298 | 268292-268442 | 280455-280462 |
| PDB 3VQ4, FBPa Site | 199871-199902 | 218283-218517 | 243079-243180 | 254299-254349 | 268443-268593 | 280463-280470 |
| PDB 3VQ4, FBPb Site | 199903-199934 | 218518-218752 | 243181-243282 | 254350-254400 | 268594-268744 | 280471-280478 |
| PDB 3VQ4, LEDGFa Site | 199935-199966 | 218753-218987 | 243283-243384 | 254401-254451 | 268745-268895 | 280479-280486 |
| PDB 3VQ4, LEDGFb Site | 199967-199998 | 218988-219222 | 243385-243486 | 254452-254502 | 268896-269046 | 280487-280494 |
| PDB 3VQ5, FBPa Site | 199999-200030 | 219223-219457 | 243487-243588 | 254503-254553 | 269047-269197 | 280495-280502 |
| PDB 3VQ5, FBPb Site | 200031-200062 | 219458-219692 | 243589-243690 | 254554-254604 | 269198-269348 | 280503-280510 |
| PDB 3VQ5, LEDGFa Site | 200063-200094 | 219693-219927 | 243691-243792 | 254605-254655 | 269349-269499 | 280511-280518 |
| PDB 3VQ5, LEDGFb Site | 200095-200126 | 219928-220162 | 243793-243894 | 254656-254706 | 269500-269650 | 280519-280526 |
| PDB 3VQ7, FBPa Site | 200127-200158 | 220163-220397 | 243895-243996 | 254707-254757 | 269651-269801 | 280527-280534 |
| PDB 3VQ7, FBPb Site | 200159-200190 | 220398-220632 | 243997-244098 | 254758-254808 | 269802-269952 | 280535-280542 |
| PDB 3VQ7, LEDGFa Site | 200191-200222 | 220633-220867 | 244099-244200 | 254809-254859 | 269953-270103 | 280543-280550 |
| PDB 3VQ7, LEDGFb Site | 200223-200254 | 220868-221102 | 244201-244302 | 254860-254910 | 270104-270254 | 280551-280558 |
| PDB 3VQ8, FBPa Site | 200255-200286 | 221103-221337 | 244303-244404 | 254911-254961 | 270255-270405 | 280559-280566 |
| PDB 3VQ8, FBPb Site | 200287-200318 | 221338-221572 | 244405-244506 | 254962-255012 | 270406-270556 | 280567-280574 |
| PDB 3VQ8, LEDGFa Site | 200319-200350 | 221573-221807 | 244507-244608 | 255013-255063 | 270557-270707 | 280575-280582 |
| PDB 3VQ8, LEDGFb Site | 200351-200382 | 221808-222042 | 244609-244710 | 255064-255114 | 270708-270858 | 280583-280590 |
| PDB 3VQA, FBPa Site | 200383-200414 | 222043-222277 | 244711-244812 | 255115-255165 | 270859-271009 | 280591-280598 |
| PDB 3VQA, FBPb Site | 200415-200446 | 222278-222512 | 244813-244914 | 255166-255216 | 271010-271160 | 280599-280606 |
| PDB 3VQA, LEDGFa Site | 200447-200478 | 222513-222747 | 244915-245016 | 255217-255267 | 271161-271311 | 280607-280614 |
| PDB 3VQA, LEDGFb Site | 200479-200510 | 222748-222982 | 245017-245118 | 255268-255318 | 271312-271462 | 280615-280622 |
| PDB 3VQD, FBPa Site | 200511-200542 | 222983-223217 | 245119-245220 | 255319-255369 | 271463-271613 | 280623-280630 |
| PDB 3VQD, FBPb Site | 200543-200574 | 223218-223452 | 245221-245322 | 255370-255420 | 271614-271764 | 280631-280638 |
| PDB 3VQD, LEDGFa Site | 200575-200606 | 223453-223687 | 245323-245424 | 255421-255471 | 271765-271915 | 280639-280646 |
| PDB 3VQD, LEDGFb Site | 200607-200638 | 223688-223922 | 245425-245526 | 255472-255522 | 271916-272066 | 280647-280654 |
| PDB 3VQE, FBPa Site | 200639-200670 | 223923-224157 | 245527-245628 | 255523-255573 | 272067-272217 | 280655-280662 |
| PDB 3VQE, FBPb Site | 200671-200702 | 224158-224392 | 245629-245730 | 255574-255624 | 272218-272368 | 280663-280670 |
| PDB 3VQE, LEDGFa Site | 200703-200734 | 224393-224627 | 245731-245832 | 255625-255675 | 272369-272519 | 280671-280678 |
| PDB 3VQE, LEDGFb Site | 200735-200766 | 224628-224862 | 245833-245934 | 255676-255726 | 272520-272670 | 280679-280686 |
| PDB 3ZCM, FBPa Site | 200767-200798 | 224863-225097 | 245935-246036 | 255727-255777 | 272671-272821 | 280687-280694 |
| PDB 3ZCM, FBPb Site | 200799-200830 | 225098-225332 | 246037-246138 | 255778-255828 | 272822-272972 | 280695-280702 |
| PDB 3ZCM, LEDGFa Site | 200831-200862 | 225333-225567 | 246139-246240 | 255829-255879 | 272973-273123 | 280703-280710 |
| PDB 3ZCM, LEDGFb Site | 200863-200894 | 225568-225802 | 246241-246342 | 255880-255930 | 273124-273274 | 280711-280718 |
| PDB 3ZCM, Y3a Site | 200895-200926 | 225803-226037 | 246343-246444 | 255931-255981 | 273275-273425 | 280719-280726 |
| PDB 3ZCM, Y3b Site | 200927-200958 | 226038-226272 | 246445-246546 | 255982-256032 | 273426-273576 | 280727-280734 |
| PDB 3ZSO, FBPa Site | 200959-200990 | 226273-226507 | 246547-246648 | 256033-256083 | 273577-273727 | 280735-280742 |
| PDB 3ZSO, FBPb Site | 200991-201022 | 226508-226742 | 246649-246750 | 256084-256134 | 273728-273878 | 280743-280750 |
| PDB 3ZSO, LEDGFa Site | 201023-201054 | 226743-226977 | 246751-246852 | 256135-256185 | 273879-274029 | 280751-280758 |
| PDB 3ZSO, LEDGFb Site | 201055-201086 | 226978-227212 | 246853-246954 | 256186-256236 | 274030-274180 | 280759-280766 |
| PDB 3ZSO, Y3a Site | 201087-201118 | 227213-227447 | 246955-247056 | 256237-256287 | 274181-274331 | 280767-280774 |
| PDB 3ZSO, Y3b Site | 201119-201150 | 227448-227682 | 247057-247158 | 256288-256338 | 274332-274482 | 280775-280782 |
| PDB 3ZSW, FBPa Site | 201151-201182 | 227683-227917 | 247159-247260 | 256339-256389 | 274483-274633 | 280783-280790 |
| PDB 3ZSW, FBPb Site | 201183-201214 | 227918-228152 | 247261-247362 | 256390-256440 | 274634-274784 | 280791-280798 |
| PDB 3ZSW, LEDGFa Site | 201215-201246 | 228153-228387 | 247363-247464 | 256441-256491 | 274785-274935 | 280799-280806 |
| PDB 3ZSW, LEDGFb Site | 201247-201278 | 228388-228622 | 247465-247566 | 256492-256542 | 274936-275086 | 280807-280814 |
| PDB 3ZSW, Y3a Site | 201279-201310 | 228623-228857 | 247567-247668 | 256543-256593 | 275087-275237 | 280815-280822 |
| PDB 3ZSW, Y3b Site | 201311-201342 | 228858-229092 | 247669-247770 | 256594-256644 | 275238-275388 | 280823-280830 |
| PDB 3ZT1, FBPa Site | 201343-201374 | 229093-229327 | 247771-247872 | 256645-256695 | 275389-275539 | 280831-280838 |
| PDB 3ZT1, FBPb Site | 201375-201406 | 229328-229562 | 247873-247974 | 256696-256746 | 275540-275690 | 280839-280846 |
| PDB 3ZT1, LEDGFa Site | 201407-201438 | 229563-229797 | 247975-248076 | 256747-256797 | 275691-275841 | 280847-280854 |
| PDB 3ZT1, LEDGFb Site | 201439-201470 | 229798-230032 | 248077-248178 | 256798-256848 | 275842-275992 | 280855-280862 |
| PDB 3ZT1, Y3a Site | 201471-201502 | 230033-230267 | 248179-248280 | 256849-256899 | 275993-276143 | 280863-280870 |
| PDB 3ZT1, Y3b Site | 201503-201534 | 230268-230502 | 248281-248382 | 256900-256950 | 276144-276294 | 280871-280878 |
| PDB 3ZT2, FBPa Site | 201535-201566 | 230503-230737 | 248383-248484 | 256951-257001 | 276295-276445 | 280879-280886 |
| PDB 3ZT2, FBPb Site | 201567-201598 | 230738-230972 | 248485-248586 | 257002-257052 | 276446-276596 | 280887-280894 |
| PDB 3ZT2, LEDGFa Site | 201599-201630 | 230973-231207 | 248587-248688 | 257053-257103 | 276597-276747 | 280895-280902 |
| PDB 3ZT2, LEDGFb Site | 201631-201662 | 231208-231442 | 248689-248790 | 257104-257154 | 276748-276898 | 280903-280910 |
| PDB 3ZT2, Y3a Site | 201663-201694 | 231443-231677 | 248791-248892 | 257155-257205 | 276899-277049 | 280911-280918 |
| PDB 3ZT2, Y3b Site | 201695-201726 | 231678-231912 | 248893-248994 | 257206-257256 | 277050-277200 | 280919-280926 |
| PDB 3ZT3, FBPa Site | 201727-201758 | 231913-232147 | 248995-249096 | 257257-257307 | 277201-277351 | 280927-280934 |
| PDB 3ZT3, FBPb Site | 201759-201790 | 232148-232382 | 249097-249198 | 257308-257358 | 277352-277502 | 280935-280942 |
| PDB 3ZT3, LEDGFa Site | 201791-201822 | 232383-232617 | 249199-249300 | 257359-257409 | 277503-277653 | 280943-280950 |
| PDB 3ZT3, LEDGFb Site | 201823-201854 | 232618-232852 | 249301-249402 | 257410-257460 | 277654-277804 | 280951-280958 |
| PDB 3ZT3, Y3a Site | 201855-201886 | 232853-233087 | 249403-249504 | 257461-257511 | 277805-277955 | 280959-280966 |
| PDB 3ZT3, Y3b Site | 201887-201918 | 233088-233322 | 249505-249606 | 257512-257562 | 277956-278106 | 280967-280974 |
| PDB 3ZT4, FBPa Site | 201919-201950 | 233323-233557 | 249607-249708 | 257563-257613 | 278107-278257 | 280975-280982 |
| PDB 3ZT4, FBPb Site | 201951-201982 | 233558-233792 | 249709-249810 | 257614-257664 | 278258-278408 | 280983-280990 |
| PDB 3ZT4, LEDGFa Site | 201983-202014 | 233793-234027 | 249811-249912 | 257665-257715 | 278409-278559 | 280991-280998 |
| PDB 3ZT4, LEDGFb Site | 202015-202046 | 234028-234262 | 249913-250014 | 257716-257766 | 278560-278710 | 280999-281006 |
| PDB 3ZT4, Y3a Site | 202047-202078 | 234263-234497 | 250015-250116 | 257767-257817 | 278711-278861 | 281007-281014 |
| PDB 3ZT4, Y3b Site | 202079-202110 | 234498-234732 | 250117-250218 | 257818-257868 | 278862-279012 | 281015-281022 |
| PDB 4AHR, FBPa Site | 202111-202142 | 234733-234967 | 250219-250320 | 257869-257919 | 279013-279163 | 281023-281030 |
| PDB 4AHR, FBPb Site | 202143-202174 | 234968-235202 | 250321-250422 | 257920-257970 | 279164-279314 | 281031-281038 |
| PDB 4AHR, LEDGFa Site | 202175-202206 | 235203-235437 | 250423-250524 | 257971-258021 | 279315-279465 | 281039-281046 |
| PDB 4AHR, LEDGFb Site | 202207-202238 | 235438-235672 | 250525-250626 | 258022-258072 | 279466-279616 | 281047-281054 |
| PDB 4AHR, Y3a Site | 202239-202270 | 235673-235907 | 250627-250728 | 258073-258123 | 279617-279767 | 281055-281062 |
| PDB 4AHR, Y3b Site | 202271-202302 | 235908-236142 | 250729-250830 | 258124-258174 | 279768-279918 | 281063-281070 |
| COMPLETION | 78% | 1% | 0% | 0% | 0% | 0% |
Experiments 91 to 96 (AD Vina), 79% completed
Experiments 91 through 96 (AD Vina Experiments 43 to 48) target allosteric sites of HIV integrase. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M) and Maybridge with AD Vina against various structures from the PDB. These experiments complement Experiments 47 - 51, and the resulting data will help discriminate receptor structures when many are available for a virtual screening.
| AD Exp. 91 AD Vina Exp. 43 | AD Exp. 92 AD Vina Exp. 44 | AD Exp. 93 AD Vina Exp. 45 | AD Exp. 94 AD Vina Exp. 46 | AD Exp. 95 AD Vina Exp. 47 | AD Exp. 96 AD Vina Exp. 48 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 3NF6_A, FBP Site | 168745-168776 | 170345-170579 | 182095-182196 | 187195-187245 | 189745-189895 | 197295-197302 |
| PDB 3NF6_B, FBP Site | 168777-168808 | 170580-170814 | 182197-182298 | 187246-187296 | 189896-190046 | 197303-197310 |
| PDB 3NF7_A, FBP Site | 168809-168840 | 170815-171049 | 182299-182400 | 187297-187347 | 190047-190197 | 197311-197318 |
| PDB 3NF7_B, FBP Site | 168841-168872 | 171050-171284 | 182401-182502 | 187348-187398 | 190198-190348 | 197319-197326 |
| PDB 3NF8_A, FBP Site | 168873-168904 | 171285-171519 | 182503-182604 | 187399-187449 | 190349-190499 | 197327-197334 |
| PDB 3NF9_A, FBP Site | 168905-168936 | 171520-171754 | 182605-182706 | 187450-187500 | 190500-190650 | 197335-197342 |
| PDB 3NF9_B, FBP Site | 168937-168968 | 171755-171989 | 182707-182808 | 187501-187551 | 190651-190801 | 197343-197350 |
| PDB 3NFA_A, FBP Site | 168969-169000 | 171990-172224 | 182809-182910 | 187552-187602 | 190802-190952 | 197351-197358 |
| PDB 3NFA_B, FBP Site | 169001-169032 | 172225-172459 | 182911-183012 | 187603-187653 | 190953-191103 | 197359-197366 |
| PDB 3VQ8_B, FBP Site | 169033-169064 | 172460-172694 | 183013-183114 | 187654-187704 | 191104-191254 | 197367-197374 |
| PDB 3ZCM_A, FBP Site | 169065-169096 | 172695-172929 | 183115-183216 | 187705-187755 | 191255-191405 | 197375-197382 |
| PDB 3ZSO_B, FBP Site | 169097-169128 | 172930-173164 | 183217-183318 | 187756-187806 | 191406-191556 | 197383-197390 |
| PDB 3ZSW_B, FBP Site | 169129-169160 | 173165-173399 | 183319-183420 | 187807-187857 | 191557-191707 | 197391-197398 |
| PDB 3ZT1_A, FBP Site | 169161-169192 | 173400-173634 | 183421-183522 | 187858-187908 | 191708-191858 | 197399-197406 |
| PDB 3ZT2_A, FBP Site | 169193-169224 | 173635-173869 | 183523-183624 | 187909-187959 | 191859-192009 | 197407-197414 |
| PDB 3ZT3_A, FBP Site | 169225-169256 | 173870-174104 | 183625-183726 | 187960-188010 | 192010-192160 | 197415-197422 |
| PDB 3ZT4_B, FBP Site | 169257-169288 | 174105-174339 | 183727-183828 | 188011-188061 | 192161-192311 | 197423-197430 |
| PDB 3AO1, LEDGF Site | 169289-169320 | 174340-174574 | 183829-183930 | 188062-188112 | 192312-192462 | 197431-197438 |
| PDB 3AO2, LEDGF Site | 169321-169352 | 174575-174809 | 183931-184032 | 188113-188163 | 192463-192613 | 197439-197446 |
| PDB 3NF6_B, LEDGF Site | 169353-169384 | 174810-175044 | 184033-184134 | 188164-188214 | 192614-192764 | 197447-197454 |
| PDB 3NF7_A, LEDGF Site | 169385-169416 | 175045-175279 | 184135-184236 | 188215-188265 | 192765-192915 | 197455-197462 |
| PDB 3NF7_B, LEDGF Site | 169417-169448 | 175280-175514 | 184237-184338 | 188266-188316 | 192916-193066 | 197463-197470 |
| PDB 3NF8_B, LEDGF Site | 169449-169480 | 175515-175749 | 184339-184440 | 188317-188367 | 193067-193217 | 197471-197478 |
| PDB 3NF9_A, LEDGF Site | 169481-169512 | 175750-175984 | 184441-184542 | 188368-188418 | 193218-193368 | 197479-197486 |
| PDB 3NF9_B, LEDGF Site | 169513-169544 | 175985-176219 | 184543-184644 | 188419-188469 | 193369-193519 | 197487-197494 |
| PDB 3NFA_A, LEDGF Site | 169545-169576 | 176220-176454 | 184645-184746 | 188470-188520 | 193520-193670 | 197495-197502 |
| PDB 3NFA_B, LEDGF Site | 169577-169608 | 176455-176689 | 184747-184848 | 188521-188571 | 193671-193821 | 197503-197510 |
| PDB 3VQ4, LEDGF Site | 169609-169640 | 176690-176924 | 184849-184950 | 188572-188622 | 193822-193972 | 197511-197518 |
| PDB 3VQ5_A, LEDGF Site | 169641-169672 | 176925-177159 | 184951-185052 | 188623-188673 | 193973-194123 | 197519-197526 |
| PDB 3VQ7, LEDGF Site | 169673-169704 | 177160-177394 | 185053-185154 | 188674-188724 | 194124-194274 | 197527-197534 |
| PDB 3VQA, LEDGF Site | 169705-169736 | 177395-177629 | 185155-185256 | 188725-188775 | 194275-194425 | 197535-197542 |
| PDB 3VQD, LEDGF Site | 169737-169768 | 177630-177864 | 185257-185358 | 188776-188826 | 194426-194576 | 197543-197550 |
| PDB 3VQE_A, LEDGF Site | 169769-169800 | 177865-178099 | 185359-185460 | 188827-188877 | 194577-194727 | 197551-197558 |
| PDB 4AHR_A, LEDGF Site | 169801-169832 | 178100-178334 | 185461-185562 | 188878-188928 | 194728-194878 | 197559-197566 |
| PDB 3AO1, Y3 Site | 169833-169864 | 178335-178569 | 185563-185664 | 188929-188979 | 194879-195029 | 197567-197574 |
| PDB 3AO2, Y3 Site | 169865-169896 | 178570-178804 | 185665-185766 | 188980-189030 | 195030-195180 | 197575-197582 |
| PDB 3VQ4, Y3 Site | 169897-169928 | 178805-179039 | 185767-185868 | 189031-189081 | 195181-195331 | 197583-197590 |
| PDB 3VQ5_A, Y3 Site | 169929-169960 | 179040-179274 | 185869-185970 | 189082-189132 | 195332-195482 | 197591-197598 |
| PDB 3VQ8_B, Y3 Site | 169961-169992 | 179275-179509 | 185971-186072 | 189133-189183 | 195483-195633 | 197599-197606 |
| PDB 3VQA, Y3 Site | 169993-170024 | 179510-179744 | 186073-186174 | 189184-189234 | 195634-195784 | 197607-197614 |
| PDB 3VQD, Y3 SiteA | 170025-170056 | 179745-179979 | 186175-186276 | 189235-189285 | 195785-195935 | 197615-197622 |
| PDB 3VQE_A, Y3 Site | 170057-170088 | 179980-180214 | 186277-186378 | 189286-189336 | 195936-196086 | 197623-197630 |
| PDB 3ZCM_A, Y3 Site | 170089-170120 | 180215-180449 | 186379-186480 | 189337-189387 | 196087-196237 | 197631-197638 |
| PDB 3ZSO_B, Y3 Site | 170121-170152 | 180450-180684 | 186481-186582 | 189388-189438 | 196238-196388 | 197639-197646 |
| PDB 3ZSW_B, Y3 Site | 170153-170184 | 180685-180919 | 186583-186684 | 189439-189489 | 196389-196539 | 197647-197654 |
| PDB 3ZT1_A, Y3 Site | 170185-170216 | 180920-181154 | 186685-186786 | 189490-189540 | 196540-196690 | 197655-197662 |
| PDB 3ZT2_A, Y3 Site | 170217-170248 | 181155-181389 | 186787-186888 | 189541-189591 | 196691-196841 | 197663-197670 |
| PDB 3ZT3_A, Y3 Site | 170249-170280 | 181390-181624 | 186889-186990 | 189592-189642 | 196842-196992 | 197671-197678 |
| PDB 3ZT4_B, Y3 Site | 170281-170312 | 181625-181859 | 186991-187092 | 189643-189693 | 196993-197143 | 197679-197686 |
| PDB 4AHR_A, Y3 Site | 170313-170344 | 181860-182094 | 187093-187194 | 189694-189744 | 197144-197294 | 197687-197694 |
| COMPLETION | 100% | 99.9% | 99.7% | 100% | 66% | 6% |
Experiments 85 to 90 (AD Vina), 100% completed
Experiments 85 through 90 (AD Vina Experiments 37 to 42) target allosteric sites of HIV protease. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M) and Maybridge with AD Vina against various structures from the PDB.
| AD Exp. 85 | AD Exp. 86 | AD Exp. 87 | AD Exp. 88 | AD Exp. 89 | AD Exp. 90 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB 1ZTZ, 1F1 Site, Chain A | 127057-127088 | 129361-129595 | 146281-146382 | 153625-153675 | 157297-157447 | 168169-168176 |
| PDB 1ZTZ, 1F1 Site, Chain B | 127089-127120 | 129596-129830 | 146383-146484 | 153676-153726 | 157448-157598 | 168177-168184 |
| PDB 1ZTZ, Disulfide Site, Chain A | 127121-127152 | 129831-130065 | 146485-146586 | 153727-153777 | 157599-157749 | 168185-168192 |
| PDB 1ZTZ, Disulfide Site, Chain B | 127153-127184 | 130066-130300 | 146587-146688 | 153778-153828 | 157750-157900 | 168193-168200 |
| PDB 1ZTZ, "Ear" Site, Chain A | 127185-127216 | 130301-130535 | 146689-146790 | 153829-153879 | 157901-158051 | 168201-168208 |
| PDB 1ZTZ, "Ear" Site, Chain B | 127217-127248 | 130536-130770 | 146791-146892 | 153880-153930 | 158052-158202 | 168209-168216 |
| PDB 1ZTZ, 4d9 and DMSO Sites, Chain A | 127249-127280 | 130771-131005 | 146893-146994 | 153931-153981 | 158203-158353 | 168217-168224 |
| PDB 1ZTZ, 4d9 and DMSO Sites, Chain B | 127281-127312 | 131006-131240 | 146995-147096 | 153982-154032 | 158354-158504 | 168225-168232 |
| PDB 3I8W Monomer, 1F1 Site | 127313-127344 | 131241-131475 | 147097-147198 | 154033-154083 | 158505-158655 | 168233-168240 |
| PDB 3I8W Monomer, Disulfide Site | 127345-127376 | 131476-131710 | 147199-147300 | 154084-154134 | 158656-158806 | 168241-168248 |
| PDB 3I8W Monomer, "Ear" Site | 127377-127408 | 131711-131945 | 147301-147402 | 154135-154185 | 158807-158957 | 168249-168256 |
| PDB 3I8W Monomer, 4d9 and DMSO Sites | 127409-127440 | 131946-132180 | 147403-147504 | 154186-154236 | 158958-159108 | 168257-168264 |
| PDB 3KF0, 1F1 Site, Chain A | 127441-127472 | 132181-132415 | 147505-147606 | 154237-154287 | 159109-159259 | 168265-168272 |
| PDB 3KF0, 1F1 Site, Chain B | 127473-127504 | 132416-132650 | 147607-147708 | 154288-154338 | 159260-159410 | 168273-168280 |
| PDB 3KF0, Disulfide Site, Chain A | 127505-127536 | 132651-132885 | 147709-147810 | 154339-154389 | 159411-159561 | 168281-168288 |
| PDB 3KF0, Disulfide Site, Chain B | 127537-127568 | 132886-133120 | 147811-147912 | 154390-154440 | 159562-159712 | 168289-168296 |
| PDB 3KF0, "Ear" Site, Chain A | 127569-127600 | 133121-133355 | 147913-148014 | 154441-154491 | 159713-159863 | 168297-168304 |
| PDB 3KF0, "Ear" Site, Chain B | 127601-127632 | 133356-133590 | 148015-148116 | 154492-154542 | 159864-160014 | 168305-168312 |
| PDB 3KF0, 4d9 and DMSO Sites, Chain A | 127633-127664 | 133591-133825 | 148117-148218 | 154543-154593 | 160015-160165 | 168313-168320 |
| PDB 3KF0, 4d9 and DMSO Sites, Chain B | 127665-127696 | 133826-134060 | 148219-148320 | 154594-154644 | 160166-160316 | 168321-168328 |
| PDB 3KFN, 1F1 Site, Chain A | 127697-127728 | 134061-134295 | 148321-148422 | 154645-154695 | 160317-160467 | 168329-168336 |
| PDB 3KFN, 1F1 Site, Chain B | 127729-127760 | 134296-134530 | 148423-148524 | 154696-154746 | 160468-160618 | 168337-168344 |
| PDB 3KFN, Disulfide Site, Chain A | 127761-127792 | 134531-134765 | 148525-148626 | 154747-154797 | 160619-160769 | 168345-168352 |
| PDB 3KFN, Disulfide Site, Chain B | 127793-127824 | 134766-135000 | 148627-148728 | 154798-154848 | 160770-160920 | 168353-168360 |
| PDB 3KFN, "Ear" Site, Chain A | 127825-127856 | 135001-135235 | 148729-148830 | 154849-154899 | 160921-161071 | 168361-168368 |
| PDB 3KFN, "Ear" Site, Chain B | 127857-127888 | 135236-135470 | 148831-148932 | 154900-154950 | 161072-161222 | 168369-168376 |
| PDB 3KFN, 4d9 and DMSO Sites, Chain A | 127889-127920 | 135471-135705 | 148933-149034 | 154951-155001 | 161223-161373 | 168377-168384 |
| PDB 3KFN, 4d9 and DMSO Sites, Chain B | 127921-127952 | 135706-135940 | 149035-149136 | 155002-155052 | 161374-161524 | 168385-168392 |
| PDB 3KFP Monomer, 1F1 Site | 127953-127984 | 135941-136175 | 149137-149238 | 155053-155103 | 161525-161675 | 168393-168400 |
| PDB 3KFP Monomer, Disulfide Site | 127985-128016 | 136176-136410 | 149239-149340 | 155104-155154 | 161676-161826 | 168401-168408 |
| PDB 3KFP Monomer, "Ear" Site | 128017-128048 | 136411-136645 | 149341-149442 | 155155-155205 | 161827-161977 | 168409-168416 |
| PDB 3KFP Monomer, 4d9 and DMSO Sites | 128049-128080 | 136646-136880 | 149443-149544 | 155206-155256 | 161978-162128 | 168417-168424 |
| PDB 3KFR, 1F1 Site, Chain A | 128081-128112 | 136881-137115 | 149545-149646 | 155257-155307 | 162129-162279 | 168425-168432 |
| PDB 3KFR, 1F1 Site, Chain B | 128113-128144 | 137116-137350 | 149647-149748 | 155308-155358 | 162280-162430 | 168433-168440 |
| PDB 3KFR, Disulfide Site, Chain A | 128145-128176 | 137351-137585 | 149749-149850 | 155359-155409 | 162431-162581 | 168441-168448 |
| PDB 3KFR, Disulfide Site, Chain B | 128177-128208 | 137586-137820 | 149851-149952 | 155410-155460 | 162582-162732 | 168449-168456 |
| PDB 3KFR, "Ear" Site, Chain A | 128209-128240 | 137821-138055 | 149953-150054 | 155461-155511 | 162733-162883 | 168457-168464 |
| PDB 3KFR, "Ear" Site, Chain B | 128241-128272 | 138056-138290 | 150055-150156 | 155512-155562 | 162884-163034 | 168465-168472 |
| PDB 3KFR, 4d9 and DMSO Sites, Chain A | 128273-128304 | 138291-138525 | 150157-150258 | 155563-155613 | 163035-163185 | 168473-168480 |
| PDB 3KFR, 4d9 and DMSO Sites, Chain B | 128305-128336 | 138526-138760 | 150259-150360 | 155614-155664 | 163186-163336 | 168481-168488 |
| PDB 3KFS, 1F1 Site, Chain A | 128337-128368 | 138761-138995 | 150361-150462 | 155665-155715 | 163337-163487 | 168489-168496 |
| PDB 3KFS, 1F1 Site, Chain B | 128369-128400 | 138996-139230 | 150463-150564 | 155716-155766 | 163488-163638 | 168497-168504 |
| PDB 3KFS, Disulfide Site, Chain A | 128401-128432 | 139231-139465 | 150565-150666 | 155767-155817 | 163639-163789 | 168505-168512 |
| PDB 3KFS, Disulfide Site, Chain B | 128433-128464 | 139466-139700 | 150667-150768 | 155818-155868 | 163790-163940 | 168513-168520 |
| PDB 3KFS, "Ear" Site, Chain A | 128465-128496 | 139701-139935 | 150769-150870 | 155869-155919 | 163941-164091 | 168521-168528 |
| PDB 3KFS, "Ear" Site, Chain B | 128497-128528 | 139936-140170 | 150871-150972 | 155920-155970 | 164092-164242 | 168529-168536 |
| PDB 3KFS, 4d9 and DMSO Sites, Chain A | 128529-128560 | 140171-140405 | 150973-151074 | 155971-156021 | 164243-164393 | 168537-168544 |
| PDB 3KFS, 4d9 and DMSO Sites, Chain B | 128561-128592 | 140406-140640 | 151075-151176 | 156022-156072 | 164394-164544 | 168545-168552 |
| PDB 3T11, 1F1 Site, Chain A | 128593-128624 | 140641-140875 | 151177-151278 | 156073-156123 | 164545-164695 | 168553-168560 |
| PDB 3T11, 1F1 Site, Chain B | 128625-128656 | 140876-141110 | 151279-151380 | 156124-156174 | 164696-164846 | 168561-168568 |
| PDB 3T11, Disulfide Site, Chain A | 128657-128688 | 141111-141345 | 151381-151482 | 156175-156225 | 164847-164997 | 168569-168576 |
| PDB 3T11, Disulfide Site, Chain B | 128689-128720 | 141346-141580 | 151483-151584 | 156226-156276 | 164998-165148 | 168577-168584 |
| PDB 3T11, "Ear" Site, Chain A | 128721-128752 | 141581-141815 | 151585-151686 | 156277-156327 | 165149-165299 | 168585-168592 |
| PDB 3T11, "Ear" Site, Chain B | 128753-128784 | 141816-142050 | 151687-151788 | 156328-156378 | 165300-165450 | 168593-168600 |
| PDB 3T11, 4d9 and DMSO Sites, Chain A | 128785-128816 | 142051-142285 | 151789-151890 | 156379-156429 | 165451-165601 | 168601-168608 |
| PDB 3T11, 4d9 and DMSO Sites, Chain B | 128817-128848 | 142286-142520 | 151891-151992 | 156430-156480 | 165602-165752 | 168609-168616 |
| PDB 4DQG, 1F1 Site, Chain A | 128849-128880 | 142521-142755 | 151993-152094 | 156481-156531 | 165753-165903 | 168617-168624 |
| PDB 4DQG, 1F1 Site, Chain B | 128881-128912 | 142756-142990 | 152095-152196 | 156532-156582 | 165904-166054 | 168625-168632 |
| PDB 4DQG, Disulfide Site, Chain A | 128913-128944 | 142991-143225 | 152197-152298 | 156583-156633 | 166055-166205 | 168633-168640 |
| PDB 4DQG, Disulfide Site, Chain B | 128945-128976 | 143226-143460 | 152299-152400 | 156634-156684 | 166206-166356 | 168641-168648 |
| PDB 4DQG, "Ear" Site, Chain A | 128977-129008 | 143461-143695 | 152401-152502 | 156685-156735 | 166357-166507 | 168649-168656 |
| PDB 4DQG, "Ear" Site, Chain B | 129009-129040 | 143696-143930 | 152503-152604 | 156736-156786 | 166508-166658 | 168657-168664 |
| PDB 4DQG, 4d9 and DMSO Sites, Chain A | 129041-129072 | 143931-144165 | 152605-152706 | 156787-156837 | 166659-166809 | 168665-168672 |
| PDB 4DQG, 4d9 and DMSO Sites, Chain B | 129073-129104 | 144166-144400 | 152707-152808 | 156838-156888 | 166810-166960 | 168673-168680 |
| PDB 4E43, 1F1 Site, Chain A | 129105-129136 | 144401-144635 | 152809-152910 | 156889-156939 | 166961-167111 | 168681-168688 |
| PDB 4E43, 1F1 Site, Chain B | 129137-129168 | 144636-144870 | 152911-153012 | 156940-156990 | 167112-167262 | 168689-168696 |
| PDB 4E43, Disulfide Site, Chain A | 129169-129200 | 144871-145105 | 153013-153114 | 156991-157041 | 167263-167413 | 168697-168704 |
| PDB 4E43, Disulfide Site, Chain B | 129201-129232 | 145106-145340 | 153115-153216 | 157042-157092 | 167414-167564 | 168705-168712 |
| PDB 4E43, "Ear" Site, Chain A | 129233-129264 | 145341-145575 | 153217-153318 | 157093-157143 | 167565-167715 | 168713-168720 |
| PDB 4E43, "Ear" Site, Chain B | 129265-129296 | 145576-145810 | 153319-153420 | 157144-157194 | 167716-167866 | 168721-168728 |
| PDB 4E43, 4d9 and DMSO Sites, Chain A | 129297-129328 | 145811-146045 | 153421-153522 | 157195-157245 | 167867-168017 | 168729-168736 |
| PDB 4E43, 4d9 and DMSO Sites, Chain B | 129329-129360 | 146046-146280 | 153523-153624 | 157246-157296 | 168018-168168 | 168737-168744 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% | 100% |
Experiments 79 to 84 (AD Vina), 100% completed
Experiments 79 through 84 target (AD Vina Experiments 31 to 36) the exo site region of HIV protease; they are the same experiments as Experiments 73 - 78, but Vina is used instead of AutoDock. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M) and Maybridge with AD Vina against a crystal structure from the Stout Lab and PDB 3KF0.
| AD Exp. 79 (31) | AD Exp. 80 (32) | AD Exp. 81 (33) | AD Exp. 82 (34) | AD Exp. 83 (35) | AD Exp. 84 (36) | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB, 3KF0, Chain A | 124401-124432 | 124529-124763 | 125469-125570 | 125877-125927 | 126081-126231 | 126749-126825 |
| Stout Lab Structure, Chain A | 124433-124464 | 124764-124998 | 125571-125672 | 125928-125978 | 126232-126382 | 126826-126902 |
| PDB, 3KF0, Chain B | 124465-124496 | 124999-125233 | 125673-125774 | 125979-126029 | 126383-126533 | 126903-126979 |
| Stout Lab Structure, Chain B | 124497-124528 | 125234-125468 | 125775-125876 | 126030-126080 | 126534-126684 | 126980-127056 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% | 100% |
Experiments 73 to 78 (AutoDock), 31% completed
Experiments 73 through 78 target the exo site region of HIV protease. These experiments involve screening 6 libraries (Full NCI, Enamine, ChemBridge, Asinex, Vitas-M) and Maybridge with AutoDock against a crystal structure from the Stout Lab and PDB 3KF0.
| AD Exp. 73 | AD Exp. 74 | AD Exp. 75 | AD Exp. 76 | AD Exp. 77 | AD Exp. 78 | |
|---|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M | Maybridge |
| PDB, 3KF0, Chain A | 101653-101969 | 102921-105265 | 112301-113314 | 116357-116863 | 118385-119888 | 124401-124477 |
| Stout Lab Structure, Chain A | 101970-102286 | 105266-107610 | 113315-114328 | 116864-117370 | 119889-121392 | 124478-124554 |
| PDB, 3KF0, Chain B | 102287-102603 | 107611-109955 | 114329-115342 | 117371-117877 | 121393-122896 | 124555-124631 |
| Stout Lab Structure, Chain B | 102604-102920 | 109956-112300 | 115343-116356 | 117878-118384 | 122897-124400 | 124632-124708 |
| COMPLETION | 100% | 62% | 19% | 0% | 0% | 0% |
Experiment 72 (AutoDock), 100% Complete
Experiments 72 targets the 4d9 site of HIV-1 protease (PR). This experiment involves screening the full NCI Set against 2 crystal structures and focuses on refining future AutoDock (and AD Vina) virtual screenings. These dockings will be compared with past dockings to the full NCI Set against PR to help overall efficiency in terms of accuracy and speed. Batch summary by parameters*: Batches 97215 - 97848: usual parameters run by Dr. Perryman (ALP) Batches 97849 - 98482: default AutoDock parameters (GA*) Batches 98483 - 99116: default AutoDock parameters (GA*) on fragment structures only Batches 99117 - 99750: default AutoDock parameters Batches 99751 - 100384: default AutoDock parameters on fragment structures only Batches 100385 - 101018: default AutoDock parameters except elitism = 5 Batches 101019 - 101652: default AutoDock parameters except elitism = 10 *All batches in this experiment are done with new docking box parameters.
| Receptor Structure | ALP Parameters | Default AD Parameters (GA*) | Default AD Parameters Fragments Only(GA*) | Default AD Parameters | Default AD Parameters Fragments Only | Elitism = 5 | Elitism = 10 |
|---|---|---|---|---|---|---|---|
| PDB 3KF0 | 97215-97531 | 97849-98165 | 98483-98799 | 99117-99433 | 99751-100067 | 100385-100701 | 101019-101335 |
| Stout Lab C6-bound structure | 97532-97848 | 98166-98482 | 98800-99116 | 99434-99750 | 100068-100384 | 100702-101018 | 101336-101652 |
Status 39-71
Experiments 52 to 71 = AD Vina Exp. 11 to 30, 100% completed
Experiments 52 through 71 (AD Vina Experiments 11 to 30) target the three allosteric sites of HIV reverse transcriptase as well as the known non-nucleoside reverse trasciptase inhibitor (NNRTI) site. These experiments involve screening 5 large libraries (Full NCI, Enamine, ChemBridge, Asinex and Vitas-M) with AD Vina against several crystal structures and molecular dynamics (MD) snapshots from the McCammon Lab, UCSD. Among the crystal structures are those from our collaborators in the Arnold Lab. The MD snapshots were graciously provided by Sara Nichols, from simulations on PDB 1VRT (see the supporting information of Predictive Power of Molecular Dynamics Receptor Structures in Virtual Screening by Nichols et. al.). Positive control dockings were first performed to choose appropriate MD snapshots for each target. While performing these initial studies, different docking parameters were tested involving placement of the docking bounding box in which docking calculations are performed. Experiment summary by site: Exp. 52 - 56: incoming nucleotide binding site (INuB) of HIV-1 Reverse Transcriptase (RT) Exp. 57 - 61: knuckles binding site (KNUC) of HIV-1 Reverse Transcriptase (RT) Exp. 62 - 66: NNRTI adjacent site (NNRTIadj) of HIV-1 Reverse Trascriptase (RT) Exp. 67 - 71: NNRTI binding site (NNRTI site) of HIV-1 Reverse Transcriptase (RT)
| AD Exp. 52 Vina Exp. 11 | AD Exp. 53 Vina Exp. 12 | AD Exp. 54 Vina Exp. 13 | AD Exp. 55 Vina Exp. 14 | AD Exp. 56 Vina Exp. 15 | |
|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M |
| MD snapshot uc1_frame_963 | 63526 - 63557 | 64006 - 64240 | 67531 - 67632 | 69061 - 69111 | 69826 - 69976 |
| MD snapshot apa_frame_1633 | 63558 - 63589 | 64241 - 64475 | 67633 - 67734 | 69112 - 69162 | 69977 - 70127 |
| MD snapshot apa_frame_1598 | 63590 - 63621 | 64476 - 64710 | 67735 - 67836 | 69163 - 69213 | 70128 - 70278 |
| MD snapshot apa_frame_1693 | 63622 - 63653 | 64711 - 64945 | 67837 - 67938 | 69214 - 69264 | 70279 - 70429 |
| MD snapshot apa_frame_1649 | 63654 - 63685 | 64946 - 65180 | 67939 - 68040 | 69265 - 69315 | 70430 - 70580 |
| MD snapshot uc1_frame_455 | 63686 - 63717 | 65181 - 65415 | 68041 - 68142 | 69316 - 69366 | 70581 - 70731 |
| MD snapshot uc1_frame_1241 | 63718 - 63749 | 65416 - 65650 | 68143 - 68244 | 69367 - 69417 | 70732 - 70882 |
| MD snapshot uc1_frame_1198 | 63750 - 63781 | 65651 - 65885 | 68245 - 68346 | 69418 - 69468 | 70883 - 71033 |
| PDB 1VRU, w/NNRTI* | 63782 - 63813 | 65886 - 66120 | 68347 - 68448 | 69469 - 69519 | 71034 - 71184 |
| PDB 1VRU, w/out NNRTI* | 63814 - 63845 | 66121 - 66355 | 68449 - 68550 | 69520 - 69570 | 71185 - 71335 |
| PDB 2ZD1, w/out NNRTI* | 63846 - 63877 | 66356 - 66590 | 68551 - 68652 | 69571 - 69621 | 71336 - 71486 |
| PDB 4ICL, w/out NNRTI* | 63878 - 63909 | 66591 - 66825 | 68653 - 68754 | 69622 - 69672 | 71487 - 71637 |
| PDB 4ICL, w/NNRTI* | 63910 - 63941 | 66826 - 67060 | 68755 - 68856 | 69673 - 69723 | 71638 - 71788 |
| PDB 4KFB, w/out NNRTI* | 63942 - 63973 | 67061 - 67295 | 68857 - 68958 | 69724 - 69774 | 71789 - 71939 |
| PDB 4KFB, w/NNRTI* | 63974 - 64005 | 67296 - 67530 | 68959 - 69060 | 69775 - 69825 | 71940 - 72090 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% |
| AD Exp. 57 AD Vina Exp. 16 | AD Exp. 58 AD Vina Exp. 17 | AD Exp. 59 AD Vina Exp. 18 | AD Exp. 60 AD Vina Exp. 19 | AD Exp. 61 AD Vina Exp. 20 | |
|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M |
| MD snapshot apa_frame_1701 | 72091 - 72122 | 72411 - 72645 | 74761 - 74862 | 75781 - 75831 | 76291 - 76441 |
| MD snapshot apa_frame_1702 | 72123 - 72154 | 72646 - 72880 | 74863 - 74964 | 75832 - 75882 | 76442 - 76592 |
| PDB 1FK9, w/NNRTI* | 72155 - 72186 | 72881 - 73115 | 74965 - 75066 | 75883 - 75933 | 76593 - 76743 |
| PDB 1FK9, w/out NNRTI* | 72187 - 72218 | 73116 - 73350 | 75067 - 75168 | 75934 - 75984 | 76744 - 76894 |
| PDB 4IFY, w/out NNRTI* [KNUC and INuB sites centered] |
72219 - 72250 | 73351 - 73585 | 75169 - 75270 | 75985 - 76035 | 76895 - 77045 |
| PDB 4IFY, w/out NNRTI* [KNUC site centered] |
72251 - 72282 | 73586 - 73820 | 75271 - 75372 | 76036 - 76086 | 77046 - 77196 |
| PDB 4IFY, w/NNRTI* [KNUC and INuB sites centered] |
72283 - 72314 | 73821 - 74055 | 75373 - 75474 | 76087 - 76137 | 77197 - 77347 |
| PDB 4KFB, w/out NNRTI* [KNUC and INuB sites centered] |
72315 - 72346 | 74056 - 74290 | 75475 - 75576 | 76138 - 76188 | 77348 - 77498 |
| PDB 4KFB, w/out NNRTI* [KNUC site centered] |
72347 - 72378 | 74291 - 74525 | 75577 - 75678 | 76189 - 76239 | 77499 - 77649 |
| PDB 4KFB, w/NNRTI* [KNUC and INuB sites centered] |
72379 - 72410 | 74526 - 74760 | 75679 - 75780 | 76240 - 76290 | 77650 - 77800 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% |
| AD Exp. 62 AD Vina Exp. 21 | AD Exp. 63 AD Vina Exp. 22 | AD Exp. 64 AD Vina Exp. 23 | AD Exp. 65 AD Vina Exp. 24 | AD Exp. 66 AD Vina Exp. 25 | |
|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M |
| PDB 2ZD1, w/NNRTI* | 77801 - 77832 | 77897 - 78131 | 78602 - 78703 | 78908 - 78958 | 79061 - 79211 |
| PDB 4IFY, w/NNRTI* | 77833 - 77864 | 78132 - 78366 | 78704 - 78805 | 78959 - 79009 | 79212 - 79362 |
| PDB 4KFB, w/NNRTI* | 77865 - 77896 | 78367 - 78601 | 78806 - 78907 | 79010 - 79060 | 79363 - 79513 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% |
| AD Exp. 67 AD Vina Exp. 26 | AD Exp. 68 AD Vina Exp. 27 | AD Exp. 69 AD Vina Exp. 28 | AD Exp. 70 AD Vina Exp. 29 | AD Exp. 71 AD Vina Exp. 30 | |
|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M |
| MD snapshot uc1_frame_963 | 79514 - 79545 | 80506 - 80740 | 87791 - 87892 | 90953 - 91003 | 92534 - 92684 |
| MD snapshot uc1_frame_209 | 79546 - 79577 | 80741 - 80975 | 87893 - 87994 | 91004 - 91054 | 92685 - 92835 |
| MD snapshot uc1_frame_1452 | 79578 - 79609 | 80976 - 81210 | 87995 - 88096 | 91055 - 91105 | 92836 - 92986 |
| MD snapshot apa_frame_1701 | 79610 - 79641 | 81211 - 81445 | 88097 - 88198 | 91106 - 91156 | 92987 - 93137 |
| MD snapshot uc1_frame_1781 | 79642 - 79673 | 81446 - 81680 | 88199 - 88300 | 91157 - 91207 | 93138 - 93288 |
| MD snapshot uc1_frame_326 | 79674 - 79705 | 81681 - 81915 | 88301 - 88402 | 91208 - 91258 | 93289 - 93439 |
| MD snapshot uc1_frame_63 | 79706 - 79737 | 81916 - 82150 | 88403 - 88504 | 91259 - 91309 | 93440 - 93590 |
| MD snapshot uc1_frame_693 | 79738 - 79769 | 82151 - 82385 | 88505 - 88606 | 91310 - 91360 | 93591 - 93741 |
| MD snapshot uc1_frame_1076 | 79770 - 79801 | 82386 - 82620 | 88607 - 88708 | 91361 - 91411 | 93742 - 93892 |
| MD snapshot uc1_frame_978 | 79802 - 79833 | 82621 - 82855 | 88709 - 88810 | 91412 - 91462 | 93893 - 94043 |
| MD snapshot uc1_frame_1038 | 79834 - 79865 | 82856 - 83090 | 88811 - 88912 | 91463 - 91513 | 94044 - 94194 |
| MD snapshot apa_frame_1301 | 79866 - 79897 | 83091 - 83325 | 88913 - 89014 | 91514 - 91564 | 94195 - 94345 |
| MD snapshot uc1_frame_205 | 79898 - 79929 | 83326 - 83560 | 89015 - 89116 | 91565 - 91615 | 94346 - 94496 |
| MD snapshot apa_frame_1845 | 79930 - 79961 | 83561 - 83795 | 89117 - 89218 | 91616 - 91666 | 94497 - 94647 |
| MD snapshot uc1_frame_1062 | 79962 - 79993 | 83796 - 84030 | 89219 - 89320 | 91667 - 91717 | 94648 - 94798 |
| MD snapshot uc1_frame_455 | 79994 - 80025 | 84031 - 84265 | 89321 - 89422 | 91718 - 91768 | 94799 - 94949 |
| MD snapshot uc1_frame_128 | 80026 - 80057 | 84266 - 84500 | 89423 - 89524 | 91769 - 91819 | 94950 - 95100 |
| MD snapshot uc1_frame_572 | 80058 - 80089 | 84501 - 84735 | 89525 - 89626 | 91820 - 91870 | 95101 - 95251 |
| MD snapshot uc1_frame_653 | 80090 - 80121 | 84736 - 84970 | 89627 - 89728 | 91871 - 91921 | 95252 - 95402 |
| MD snapshot apa_frame_1628 | 80122 - 80153 | 84971 - 85205 | 89729 - 89830 | 91922 - 91972 | 95403 - 95553 |
| MD snapshot uc1_frame_1198 | 80154 - 80185 | 85206 - 85440 | 89831 - 89932 | 91973 - 92023 | 95554 - 95704 |
| PDB 1BQM | 80186 - 80217 | 85441 - 85675 | 89933 - 90034 | 92024 - 92074 | 95705 - 95855 |
| PDB 1EP4 | 80218 - 80249 | 85676 - 85910 | 90035 - 90136 | 92075 - 92125 | 95856 - 96006 |
| PDB 1FK9 | 80250 - 80281 | 85911 - 86145 | 90137 - 90238 | 92126 - 92176 | 96007 - 96157 |
| PDB 1KLM | 80282 - 80313 | 86146 - 86380 | 90239 - 90340 | 92177 - 92227 | 96158 - 96308 |
| PDB 1RT1 | 80314 - 80345 | 86381 - 86615 | 90341 - 90442 | 92228 - 92278 | 96309 - 96459 |
| PDB 1RT4 | 80346 - 80377 | 86616 - 86850 | 90443 - 90544 | 92279 - 92329 | 96460 - 96610 |
| PDB 1VRT | 80378 - 80409 | 86851 - 87085 | 90545 - 90646 | 92330 - 92380 | 96611 - 96761 |
| PDB 1VRU | 80410 - 80441 | 87086 - 87320 | 90647 - 90748 | 92381 - 92431 | 96762 - 96912 |
| PDB 2ZD1 | 80442 - 80473 | 87321 - 87555 | 90749 - 90850 | 92432 - 92482 | 96913 - 97063 |
| PDB 4IFY | 80474 - 80505 | 87556 - 87790 | 90851 - 90952 | 92483 - 92533 | 97064 - 97214 |
| COMPLETION | 99.7% | 99.9% | 99.9% | 100% | 100% |
Experiments 47 to 51 = AD Vina Exp. 6 to 10, 100% completed
Experiments 47 through 51 (that is, AD Vina Experiments 6 to 10) target the three recently-discovered allosteric sites of HIV integrase. These experiments involve screening ~ 5.6 million compounds against 30 models of different crystal structures of HIV integrase (or "IN"). Each of these crystal structures (3-D, atomically-detailed maps of the location of all of the atoms of the protein) involved an allosteric inhibitor bound to at least one of these three allosteric sites (but some of these allosteric inhibitors were fragments that bound to two or three of these different allosteric sites simultaneously). Thus, we are screening compounds against allosteric-inhibitor-induced conformations (or shapes) of HIV integrase. HIV integrase is one of the three viral enzymes that must work properly in order for HIV to replicate itself and spread. Specifically, HIV integrase processes and then permanently attaches (or "integrates") the viral cDNA into the human genomic DNA of the cells that HIV infects. To learn what "allosteric inhibitors are," see page 4 of Volume 11 of our FightAIDS@Home Newsletter. In these 5 experiments with AD Vina, we will search for new compounds that can bind to at least one of the three allosteric sites of HIV integrase, which are called the LEDGF site, the FBP Site (for Fragment Binding Pocket), or the Y3 site (which is adjacent to the region underneath the "140s loop"). To learn more about these allosteric sites on the catalytic core domain of HIV integrase, see this paper by the Deadman group, this paper by the Scanlon group, this newer paper by the Scanlon group, or see this paper by Alan Engelman, Jacques J. Kessl, and Mamuka Kvaratskhelia. The Engelman and Kvaratskhelia labs, and several other labs at 6 different institutions around the country, recently started collaborating with the Olson lab, as part of the new NIH-funded Center called the "HIVE."
| AD Exp. 47 AD Vina Exp. 6 | AD Exp. 48 AD Vina Exp. 7 | AD Exp. 49 AD Vina Exp. 8 | AD Exp. 50 AD Vina Exp. 9 | AD Exp. 51 AD Vina Exp. 10 | |
|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M |
| FBP allosteric site of IN from PDB 3AO1 | 46396-46427 | 47356-47590 | 54406-54507 | 57466-57516 | 58996-59146 |
| FBP allosteric site of IN from PDB 3AO2 | 46428-46459 | 47591-47825 | 54508-54609 | 57517-57567 | 59147-59297 |
| FBP allosteric site of IN from PDB 3NF8_B | 46460-46491 | 47826-48060 | 54610-54711 | 57568-57618 | 59298-59448 |
| FBP allosteric site of IN from PDB 3VQ4 | 46492-46523 | 48061-48295 | 54712-54813 | 57619-57669 | 59449-59599 |
| FBP allosteric site of IN from PDB 3VQ5_A | 46524-46555 | 48296-48530 | 54814-54915 | 57670-57720 | 59600-59750 |
| FBP allosteric site of IN from PDB 3VQ7 | 46556-46587 | 48531-48765 | 54916-55017 | 57721-57771 | 59751-59901 |
| FBP allosteric site of IN from PDB 3VQA | 46588-46619 | 48766-49000 | 55018-55119 | 57772-57822 | 59902-60052 |
| FBP allosteric site of IN from PDB 3VQD | 46620-46651 | 49001-49235 | 55120-55221 | 57823-57873 | 60053-60203 |
| FBP allosteric site of IN from PDB 3VQE_A | 46652-46683 | 49236-49470 | 55222-55323 | 57874-57924 | 60204-60354 |
| FBP allosteric site of IN from PDB 4AHR_A | 46684-46715 | 49471-49705 | 55324-55425 | 57925-57975 | 60355-60505 |
| LEDGF allosteric site of IN from PDB 3NF6_A | 46716-46747 | 49706-49940 | 55426-55527 | 57976-58026 | 60506-60656 |
| LEDGF allosteric site of IN from PDB 3NF8_A | 46748-46779 | 49941-50175 | 55528-55629 | 58027-58077 | 60657-60807 |
| LEDGF allosteric site of IN from PDB 3VQ8_B | 46780-46811 | 50176-50410 | 55630-55731 | 58078-58128 | 60808-60958 |
| LEDGF allosteric site of IN from PDB 3ZCM_A | 46812-46843 | 50411-50645 | 55732-55833 | 58129-58179 | 60959-61109 |
| LEDGF allosteric site of IN from PDB 3ZSO_B | 46844-46875 | 50646-50880 | 55834-55935 | 58180-58230 | 61110-61260 |
| LEDGF allosteric site of IN from PDB 3ZSW_B | 46876-46907 | 50881-51115 | 55936-56037 | 58231-58281 | 61261-61411 |
| LEDGF allosteric site of IN from PDB 3ZT1_A | 46908-46939 | 51116-51350 | 56038-56139 | 58282-58332 | 61412-61562 |
| LEDGF allosteric site of IN from PDB 3ZT2_A | 46940-46971 | 51351-51585 | 56140-56241 | 58333-58383 | 61563-61713 |
| LEDGF allosteric site of IN from PDB 3ZT3_A | 46972-47003 | 51586-51820 | 56242-56343 | 58384-58434 | 61714-61864 |
| LEDGF allosteric site of IN from PDB 3ZT4_B | 47004-47035 | 51821-52055 | 56344-56445 | 58435-58485 | 61865-62015 |
| Y3 allosteric site of IN from PDB 3NF6_A | 47036-47067 | 52056-52290 | 56446-56547 | 58486-58536 | 62016-62166 |
| Y3 allosteric site of IN from PDB 3NF6_B | 47068-47099 | 52291-52525 | 56548-56649 | 58537-58587 | 62167-62317 |
| Y3 allosteric site of IN from PDB 3NF7_A | 47100-47131 | 52526-52760 | 56650-56751 | 58588-58638 | 62318-62468 |
| Y3 allosteric site of IN from PDB 3NF7_B | 47132-47163 | 52761-52995 | 56752-56853 | 58639-58689 | 62469-62619 |
| Y3 allosteric site of IN from PDB 3NF8_A | 47164-47195 | 52996-53230 | 56854-56955 | 58690-58740 | 62620-62770 |
| Y3 allosteric site of IN from PDB 3NF8_B | 47196-47227 | 53231-53465 | 56956-57057 | 58741-58791 | 62771-62921 |
| Y3 allosteric site of IN from PDB 3NF9_A | 47228-47259 | 53466-53700 | 57058-57159 | 58792-58842 | 62922-63072 |
| Y3 allosteric site of IN from PDB 3NF9_B | 47260-47291 | 53701-53935 | 57160-57261 | 58843-58893 | 63073-63223 |
| Y3 allosteric site of IN from PDB 3NFA_A | 47292-47323 | 53936-54170 | 57262-57363 | 58894-58944 | 63224-63374 |
| Y3 allosteric site of IN from PDB 3NFA_B | 47324-47355 | 54171-54405 | 57364-57465 | 58945-58995 | 63375-63525 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% |
Experiments 42 to 46 = AD Vina Exp. 1 to 5, 100% completed
Experiments 42 through 46 (that is, AD Vina Experiments 1 to 5) involve screening ~ 5.6 million compounds against two models of a recently-discovered allosteric site of HIV reverse transcriptase. Reverse transcriptase (or "RT") is one of the three viral enzymes that must work properly in order for HIV to replicate itself and spread. These are the first FightAIDS@Home experiments that target the RT system. To learn what "allosteric inhibitors are," see page 4 of Volume 11 of our FightAIDS@Home Newsletter. In these first 5 experiments with AD Vina (see http://vina.scripps.edu), we will be trying to discover new compounds that can bind to the "NNRTI adjacent" allosteric site of RT. This NNRTI adjacent allosteric site, and two other recently-discovered allosteric sites on HIV reverse transcriptase, were discovered by Eddy Arnold's lab at Rutgers University. Eddy Arnold's lab, and several other labs at 6 different institutions around the country, recently started collaborating with the Olson lab, as part of the new NIH-funded Center called the "HIVE."
| AD Exp. 42 AD Vina Exp. 1 | AD Exp. 43 AD Vina Exp. 2 | AD Exp. 44 AD Vina Exp. 3 | AD Exp. 45 AD Vina Exp. 4 | AD Exp. 46 AD Vina Exp. 5 | |
|---|---|---|---|---|---|
| Receptor Structure | Full NCI | Enamine | ChemBridge | Asinex | Vitas-M |
| NNRTI Adjacent site of RT from PDB 4I7G | 45756-45787 | 45489-45723 | 45890-45991 | 46043-46093 | 46245-46395 |
| NNRTI Adjacent site of RT from PDB 4I7G with NNRTI | 45724-45755 | 45254-45488 | 45788-45889 | 45992-46042 | 46094-46244 |
| COMPLETION | 100% | 100% | 100% | 100% | 100% |
Experiment 41: 100% Completed
Experiment 41 involves screening the Enamine library of 2.345 million compounds against the two allosteric sites on the surface of HIV protease. Experiment 41 and all previous FAAH experiments were performed with the software "AutoDock" (see http://autodock.scripps.edu ). This experiment is similar to Exp. 36 (see page 2 of the Status for a description of Exp. 36), but a different, much larger library of compounds is being screened, and some new targets are being included. The first part of this experiment involves docking compounds against the two allosteric-fragment-bound crystal structures presented in our recent article in Chemical Biology and Drug Design, vol. 75: 257-268 (March 2010). Three other, brand new crystal structures from Dave Stout's lab that involve allosteric fragments bound to these two sites on the surface of HIV protease are also being used as targets. Two of these new targets are presented in a new research manuscript from the Stout lab that is currently being peer-reviewed. When this paper is accepted, we will describe these targets in more detail and provide a link to this new paper. This is by far the largest experiment we have submitted to FightAIDS@Home; it involves faah33,529 - faah45,253. The results of these calculations started arriving at TSRI on 5/15/2012.
Experiment 40: 100% Completed
Experiment 40 involves screening the ChemBridge library of 1,013,483 models of compounds against the newly-discovered allosteric binding site on HIV-1 integrase. This new allosteric binding site (which we are also targeting in Experiments 38 and 39) was discovered by Professor John J. Deadman's group, and it was described in "Structural basis for a new mechanism of inhibition of HIV-1 integrase identified by fragment screening and structure-based design," by D.I. Rhodes, T.S. Peat, J.J. Deadman, et al., published in the journal Antiviral Chemistry and Chemotherapy, 21: 155-168 (2011). The new crystal structure from this paper that contains the atomically-detailed, 3-D data on this new allosteric site is called "3NF6.pdb". We are screening compounds against this allosteric site to try to discover new, larger, more potent allosteric inhibitors of HIV-1 integrase. It is hoped that these new allosteric inhibitors of integrase will be effective at disabling the current drug-resistant mutant superbugs of HIV integrase. For more information about this new allosteric site, see Volume 10 of the FightAIDS@Home newsletter or our recent World AIDS Day webinar (both are linked at the top of the homepage for this site). Similar to Experiment 39, in Experiment 40 we are screening one million compounds against the new allosteric site on HIV-1 integrase using two slightly different docking approaches: in the first half of these calculations, we are using the smaller dimensions of the "grid box" (the region that the compounds are allowed to explore during the docking calculations) that produced the best results in the "positive control" docking calculations that reproduced the known binding mode of this new allosteric fragment (see the figures in Volume 10 of the FAAH Newsletter, page 8). In the second half of these calculations, we are using a larger grid box, to try to find even larger allosteric inhibitors that can bind strongly with both the allosteric site and other sub-pockets that are adjacent to it. This experiment involves faah31,501 - faah33,528. These results began arriving at TSRI on 3/24/2012, and the experiment finished 5/29/2012.
Experiment 39: 100% Completed
Experiment 39 involves screening the Enamine library of 2,345,014 compounds against the newly-discovered allosteric binding site on HIV-1 integrase. This new allosteric binding site (which we are also targeting in Experiment 38) was discovered by Professor John J. Deadman's group, and it was described in "Structural basis for a new mechanism of inhibition of HIV-1 integrase identified by fragment screening and structure-based design," by D.I. Rhodes, T.S. Peat, J.J. Deadman, et al., published in the journal Antiviral Chemistry and Chemotherapy, 21: 155-168 (2011). The new crystal structure from this paper that contains the atomically-detailed, 3-D data on this new allosteric site is called "3NF6.pdb". We are screening compounds against this allosteric site to try to discover new, larger, more potent allosteric inhibitors of HIV-1 integrase. It is hoped that these new allosteric inhibitors of integrase will be effective at disabling the current drug-resistant mutant superbugs of HIV integrase. For more information about this new allosteric site, see Volume 10 of the FightAIDS@Home newsletter or our recent World AIDS Day webinar (both are linked at the top of the homepage for this site). In Experiment 39, we are screening these 2.3 million compounds against the new allosteric site on HIV-1 integrase using two slightly different docking approaches: in the first half of these calculations, we are using the smaller dimensions of the "grid box" (the region that the compounds are allowed to explore during the docking calculations) that produced the best results in the "positive control" docking calculations that reproduced the known binding mode of this new allosteric fragment (see the figures in Volume 10 of the FAAH Newsletter, page 8). In the second half of these calculations, we are using a larger grid box, to try to find even larger allosteric inhibitors that can bind strongly with both the allosteric site and other sub-pockets that are adjacent to it. This experiment involves faah26,811 - faah31,500. These calculations began (that is, the results started arriving at TSRI) on 12/17/2011. The last batch of results arrived 5/15/2012.
Status 19-38
Experiment 38: 100% Completed
Experiment 38 involves screening the full NCI library of 316,179 compounds against the newly-discovered allosteric binding site on HIV-1 integrase. This new allosteric binding site was discovered by Professor John J. Deadman's group, and it was described in "Structural basis for a new mechanism of inhibition of HIV-1 integrase identified by fragment screening and structure-based design," by D.I. Rhodes, T.S. Peat, J.J. Deadman, et al., published in the journal Antiviral Chemistry and Chemotherapy, 21: 155-168 (2011). The new crystal structure from this paper that contains the atomically-detailed, 3-D data on this new allosteric site is called "3NF6.pdb". We are screening compounds against this allosteric site to try to discover new, larger, more potent allosteric inhibitors of HIV-1 integrase. It is hoped that these new allosteric inhibitors of integrase will be effective at disabling the current drug-resistant mutant superbugs of HIV integrase. For more information about this new allosteric site, see Volume 10 of the FightAIDS@Home newsletter (pages 7-8) or our recent World AIDS Day webinar (both are linked at the top of the homepage for this site). This experiment involves faah26,179 - faah26,810. These calculations began 12/06/2011 and ended 12/22/2011.
Experiment 37: 100% Completed
Experiment 37 involves screening the newly-updated version of the Asinex library of 360,00 compounds. 507,000 different models are used to represent these 360,000 compounds (due to the need to represent different protonation states and different tautomers that some of these compounds can form in solution). These compounds are being screened against the active site and the "eye site" of 6 different crystal structures of HIV protease. When the flaps have a semi-open conformation, then we can target both the "eye site" and the floor of the active site. But when the flaps have a closed conformation, the "eye site" is no longer accessible (which means that we will only target the traditional active site, which is where the current HIV protease drugs bind). The 1st target is the crystal structure of the wild type HIV protease with 5-nitroindole bound in the eye site. This new crystal structure from Prof. C. David Stout's lab was presented in the Supporting Information for our recent article in Chemical Biology and Drug Design, vol. 75: 257-268 (March 2010). Since this crystallographic structure has semi-open flaps, we are screening these compounds against both the eye site and the active site of this target. The 2nd target is the semi-open crystal structure of wild type HIV-1b protease from 1HHP.pdb. This crystal structure has been used in previous virtual screens that were performed by Prof. Heather Carlson's group, in which they did find a novel inhibitor of HIV protease activity. Thus, this particular crystal structure has already been proven to be useful for virtual screens that target the "eye site." The idea of targeting this eye site was first proposed by Prof. Heather Carlson's group. But in this experiment, we are screening different compounds against this crystal structure than the compounds that were used in previous screens against 1HHP.pdb. The 3rd target has only been used in one previous FightAIDS@Home experiment (i.e., Experiment 35). It is a brand new crystal structure from Assoc. Prof. C. David Stout's lab of the chimeric "FIV 6s98S" protease, which was developed by our collaborators Ying-Chuan Lin, Prof. Bruce E. Torbett, and Prof. John H. Elder, and which has a closed conformation of the flaps. A paper on this new crystal structure of FIV 6s98S protease was recently accepted for publication in Acta Crystallographica and can be found at the above link. This protease enzyme is "chimeric," because it contains 5 residues from HIV protease that were substituted into the corresponding positions in FIV protease. The 6th residue was also substituted from HIV protease, but it changed into a different residue during serial passage experiments (i.e., during directed evolution studies). This 6s98S FIV protease has HIV-like drug sensitivity profiles and is a new model system for multi-drug-resistant HIV protease. The 4th target is the crystal structure of wild type HIV-1b protease bound to the drug darunavir. This crystal structure from 2IEN.pdb has a closed conformation of the flaps. Whenever we target a crystal structure of HIV protease that has a compound bound to it, we delete that ligand before we prepare the model of the target for these docking studies (so that a new compound might be able to bind in its place). The 5th target is the crystal structure of wild type HIV-1b protease bound to the compound TL-3 and to the allosteric fragment "4d9". This crystal structure, which has closed flaps, was also from the 2010 Chemical Biology & Drug Design paper cited above. The 6th target is the crystal structure of the V82F/I84V multi-drug-resistant mutant (or "superbug") of HIV protease from 1MSN.pdb, which has closed flaps. The model of this target has one protonated aspartic acid 25 (i.e., one of the two catalytic residues), which should cause us to fish out slightly different types of ligands. The 7th target is another version of the semi-open crystal structure of wild type HIV-1b protease from 1HHP.pdb. That is, this is the same molecule as the 2nd target in this experiment, but this time the model has one protonated aspartic acid 25. This experiment involves faah22,630 - faah26,178. These calculations began 5/12/2011 and ended 12/15/2011 (except for 3 batches, which were finished on 2/07/2012).
Experiment 36: 100% Completed
Experiment 36 involves screening the full NCI library of ~ 316,000 compounds against the two allosteric sites on the surface of HIV protease. This experiment is similar to Exp. 31, but a different library of compounds is being screened, and in this experiment we are only docking compounds against the two allosteric-fragment-bound crystal structures from our recent article in Chemical Biology and Drug Design, vol. 75: 257-268 (March 2010). In addition, in this experiment we are investigating four slightly different docking protocols, to both advance the methods we use for drug discovery and to increase our probability of finding new "hits" against HIV protease. Since two different allosteric-fragment-induced structures are being used with four slightly different protocols, this experiment is composed of 8 parts, as follows: 1a) The crystal structure bound to allosteric fragment "1f1" is being screened using an "expanded grid box" that includes the 1f1 allosteric site, the 4d9 allosteric site (in the non-induced conformation = a decoy site), and several other decoy sites. The docking calculations will end according to the number of generations each compound explores in the genetic algorithm (that is, all 316,000 compounds will experience the same number of generations as they explore the target). 1b) The crystal structure bound to allosteric fragment "4d9" is being screened using an "expanded grid box" that includes the 4d9 allosteric site, the 1f1 allosteric site (in the non-induced conformation = a decoy site), and several other decoy sites. The docking calculations will end according to the number of generations each compound explores in the genetic algorithm (that is, all 316,000 ligands will experience the same number of generations as they explore the target). 2a) The crystal structure bound to allosteric fragment "1f1" is being screened using an "expanded grid box" that includes the 1f1 allosteric site, the 4d9 allosteric site (in the non-induced conformation), and several other decoy sites. The docking calculations will end according to the number of energy evaluations each ligand explores in the genetic algorithm (that is, all 316,000 compounds will experience the same number of energy evaluations as they explore the target). 2b) The crystal structure bound to allosteric fragment "4d9" is being screened using an "expanded grid box" that includes the 4d9 allosteric site, the 1f1 allosteric site (in the non-induced conformation), and several other decoy sites. The docking calculations will end according to the number of energy evaluations each compound explores in the genetic algorithm (that is, all 316,000 ligands will experience the same number of energy evaluations as they explore the target). 3a) The crystal structure bound to allosteric fragment "1f1" is being screened using a "focused grid box" that only includes the 1f1 allosteric site (that is, no decoy sites were included in the grid box that the compounds are allowed to explore). The docking calculations will end according to the number of energy evaluations each ligand explores in the genetic algorithm (that is, all 316,000 compounds will experience the same number of energy evaluations as they explore the target). 3b) The crystal structure bound to allosteric fragment "4d9" is being screened using a "focused grid box" that only includes the 4d9 allosteric site (that is, no decoy sites were included in the grid box that the compounds are allowed to explore). The docking calculations will end according to the number of energy evaluations each compound explores in the genetic algorithm (that is, all 316,000 ligands will experience the same number of energy evaluations as they explore the target). 4a) The crystal structure bound to allosteric fragment "1f1" is being screened using a "focused grid box" that only includes the 1f1 allosteric site (that is, no decoy sites were included in the grid box that the compounds are allowed to explore). The docking calculations will end according to the number of generations each ligand explores in the genetic algorithm (that is, all 316,000 compounds will experience the same number of generations as they explore the target). 4b) The crystal structure bound to allosteric fragment "4d9" is being screened using a "focused grid box" that only includes the 4d9 allosteric site (that is, no decoy sites were included in the grid box that the compounds are allowed to explore). The docking calculations will end according to the number of generations each ligand explores in the genetic algorithm (that is, all 316,000 ligands will experience the same number of generations as they explore the target). This experiment involves faah20,093 - faah22,620. These calculations began 4/16/2011 and ended 7/01/2011.
Experiment 35: 100% Completed
Experiment 35 involves screening the full NCI library of ~ 316,000 compounds against the active site of 8 different versions of HIV protease. Thus, this experiment is similar to Exp. 32, but a different library of compounds is being screened, and one new target has been added. All but two of these target conformations were generated by Dr. Alex L. Perryman's Molecular Dynamics (MD) simulations of 5 different variants of HIV protease. These 8 targets include 2 snapshots of the V82F/I84V mutant from ALP's 2004 paper in Protein Science. These 2 snapshots of a multi-drug-resistant "superbug" have semi-open conformations of the flaps, which makes these models good targets for the "eye site" that is located between the tip of a semi-open flap and the top of the wall of the active site. The 3rd target is the equilibration MD (EqMD) output for 1HSI.pdb, which is a semi-open conformation of HIV-2 protease. HIV-2 is the group of strains of HIV that are most common in Africa. We'll be targeting the "eye site" of 1HSI, as well. The 4th target is the EqMD output from 1MSN.pdb, which was created using a different crystal structure of the V82F/I84V superbug. This model has a closed conformation of the flaps, which means that we'll be targeting the floor of the active site. The 5th target also has a closed conformation of the flaps, but this EqMD output is from 2R5P.pdb, which is the wild type HIV-1c protease. HIV-1c is the group of strains of HIV that are most commonly found in Asia. The 6th target has semi-open flaps, and it is the EqMD output from 1TW7.pdb, which is a superbug with the mutations L10I/D25N/M36V/M46L/I54V/I62V/L63P/A71V/V82A/I84V/L90M. We'll be targeting the eye site of this superbug, too. The 7th target is a crystal structure of the wild type HIV protease with 5-nitroindole bound in the eye site. This new crystal structure from Prof. C. David Stout's lab was presented in the Supporting Information for our recent article in Chemical Biology and Drug Design, vol. 75: 257-268 (March 2010). This new research article of ours was recently discussed in a press release on Science Daily and in a news story on KPBS-FM. This paper was recently listed as one of the "most read papers" from Chemical Biology and Drug Design in 2010! I deleted the 5-nitroindole fragment from this structure before generating the AutoDock input file for this target. We'll be screening new fragments against this crystal structure's eye site, as well. The 8th target has never been used on FightAIDS@Home before. It is a brand new crystal structure from Assoc. Prof. C. David Stout's lab of the chimeric "FIV 6s98S" protease, which was developed by our collaborators Ying-Chuan Lin, Prof. Bruce E. Torbett, and Prof. John H. Elder. A paper on this new crystal structure of FIV 6s98S protease is currently being peer-reviewed. This protease enzyme is "chimeric," because it contains 5 residues from HIV protease that were substituted into the corresponding positions in FIV protease. The 6th residue was also substituted from HIV protease, but it changed into a different residue during serial passage experiments (i.e., during directed evolution studies performed with the presence of different HIV protease drugs). This 6s98S FIV protease has HIV-like drug sensitivity profiles and is a new model system for multi-drug-resistant HIV protease. This experiment involves faah17,565 - faah20,092. These calculations began 11/26/2010 and ended 4/25/2011.
Experiment 34: 100% Completed
Experiment 34 is our second FightAIDS@Home experiment that targets the HIV integrase system. See the description and the link listed under Experiment 33. Experiment 34 involves screening the "full National Cancer Institute's (NCI) library" of over 315,000 different compounds against our new dynamic models of the wild type, the G140S/Q148H drug-resistant mutant, and the E92Q/N155H drug-resistant mutant of HIV-1 integrase. We are trying to discover compounds that can bind to and inhibit the active site of the wild type and these two drug-resistant mutants. Since these models all have two magnesium ions in the active site, we are searching for compounds that can inhibit HIV integrase's "strand transfer reaction," which is what the fairly new drug raltegravir (Isentress) does. We are screening the NCI library of compounds against 4 different snapshots (that is, conformations or 3-D shapes) of HIV integrase: a) the conformation of wild type HIV integrase against which raltegravir docked the best (in the results published in our paper in the Journal of Molecular Biology, March 26, 2010), b) the snapshot of the G140S/Q148H drug-resistant mutant against which raltegravir docked the best in our previous studies, c) the most representative (that is, the most frequently observed) conformation of the E92Q/N155H drug-resistant mutant (according to the results of the QR Factorization method in VMD), and d) the 2nd most representative conformation of the E92Q/N155H drug-resistant mutant of HIV integrase's catalytic core domain. The best compounds from this virtual screen will be assessed in test tubes, in "strand transfer" assays being developed by our collaborator Dr. Ying-Chuan Lin in Prof. John Elder's lab at TSRI. This experiment involves faah16,199 - faah17,462. An extension to this experiment in which the NCI Diversity Set II library of compounds is being screened against these targets involves faah17,463 - faah17,466. A second extension to this experiment in which the "high pH" version of the full NCI library of compounds is being screened against these targets involves faah17,473 - faah17,564. These calculations began 10/11/2010 and ended 1/16/2011.
| AD Exp. 34 | AD Exp. 34 ext. 1 | AD Exp. 34 ext. 2 | |
|---|---|---|---|
| Receptor Structure | Full NCI | NCI Div. II | NCI High-pH |
| MD Snapshot 2-01450 of PDB 1B9D | 16199-16514 | 17463 | 17473-17495 |
| MD Snapshot 2-06000 of PDB 1B9F | 16515-16830 | 17464 | 17496-17518 |
| MD Snapshot 3-07390 of PDB 1QS4 | 16831-17146 | 17465 | 17519-17541 |
| MD Snapshot 1-02420 of PDB 1QS4 | 17147-17462 | 17466 | 17542-17564 |
| COMPLETION | 100% | 100% | 100% |
Experiment 33: 100% Completed
Experiment 33 is our first FightAIDS@Home experiment that targets the HIV integrase system. While we are busy analyzing and extending the previous experiments you helped us perform against HIV protease, FAAH will now start screening compounds against the new dynamic models of wild type HIV integrase, the E92Q/N155H drug-resistant mutant, and the G140S/Q148H drug-resistant mutant that we recently created. We published these new models as the cover article for the March 26, 2010, issue of the Journal of Molecular Biology. An image of an ensemble of conformations (shapes) of the G140S/Q148H mutant is shown at the bottom of this page. Experiment 33 involves screening the "Asinex library" of over 360,000 different fragments and slightly larger compounds against our new dynamic models of the E92Q/N155H drug-resistant mutant of HIV-1 integrase. We are trying to identify compounds that can attach to brand new binding sites on this mutant. Thus, we are both searching for new types of inhibitors and for new, non-active site regions to which inhibitors can bind. We are screening these compounds against 6 different snapshots of this mutant that had the most open conformations of the "140s loop" near the active site. This loop is known to be critical to the catalytic function of integrase. This 140s loop is located in the top, left corner of the image at the bottom of this page. Since the 140s loop likely has a closed conformation during catalysis, we are searching for fragments that can stabilize the open, likely inactive conformations of the 140s loop. In other words, we are searching for fragments that might act as allosteric inhibitors (flexibility wedges that alter the shapes and motions sampled by this very flexible loop). The best compounds from this virtual screen will be assessed in test tubes, in "3' processing" assays performed by our collaborator Dr. Ying-Chuan Lin in Prof. John Elder's lab at TSRI. This experiment involves faah13,985 - faah16,198. An extension to this experiment in which the NCI Diversity Set II library of compounds is being screened against these targets involves faah17,467 - faah17,472. These calculations began 6/03/2010 and ended 12/01/2010. Jan. 2011 update: 10 of the top-ranked compounds were purchased and are currently be tested by our collaborators at TSRI.
| AD Exp. 33 | AD Exp. 33 ext. 1 | |
|---|---|---|
| Receptor Structure | Asinex | NCI Div. II |
| MD Snapshot 1-04080 of PDB 1QS4 | 13985-14353 | 17467 |
| MD Snapshot 2-00020 of PDB 1QS4 | 14354-14722 | 17468 |
| MD Snapshot 2-00260 of PDB 1QS4 | 14723-15091 | 17469 |
| MD Snapshot 3-02740 of PDB 1QS4 | 15092-15460 | 17470 |
| MD Snapshot 3-07720 of PDB 1QS4 | 15461-15829 | 17471 |
| MD Snapshot 3-07910 of PDB 1QS4 | 15830-16198 | 17472 |
| COMPLETION | 100% | 100% |
Experiment 32: 100% Completed
Experiment 32 involves screening the Otava library of approximately 335,000 "building blocks" (i.e., fragments) against the active site of 7 different versions of HIV protease. All but one of these target conformations were generated by Dr. Alex L. Perryman's Molecular Dynamics (MD) simulations of 5 different variants of HIV protease. These 7 targets include 2 snapshots of the V82F/I84V mutant from ALP's 2004 paper in Protein Science. These 2 snapshots of a multi-drug-resistant "superbug" have semi-open conformations of the flaps, which makes these models good targets for the "eye site" that is located between the tip of a semi-open flap and the top of the wall of the active site. The 3rd target is the equilibration MD (EqMD) output for 1HSI.pdb, which is a semi-open conformation of HIV-2 protease. HIV-2 is the group of strains of HIV that are most common in Africa. We'll be targeting the "eye site" of 1HSI, as well. The 4th target is the EqMD output from 1MSN.pdb, which was created using a different crystal structure of the V82F/I84V superbug. This model has a closed conformation of the flaps, which means that we'll be targeting the floor of the active site. The 5th target also has a closed conformation of the flaps, but this EqMD output is from 2R5P.pdb, which is the wild type HIV-1c protease. HIV-1c is the group of strains of HIV that are most commonly found in Asia. The 6th target has semi-open flaps, and it is the EqMD output from 1TW7.pdb, which is a superbug with the mutations L10I/D25N/M36V/M46L/I54V/I62V/L63P/A71V/V82A/I84V/L90M. We'll be targeting the eye site of this superbug, too. The 7th target is a crystal structure of the wild type HIV protease with 5-nitroindole bound in the eye site. This new crystal structure from Prof. C. David Stout's lab was presented in the Supporting Information for our recent article in Chemical Biology and Drug Design, vol. 75: 257-268 (March 2010). This new research article of ours was recently discussed in a press release on Science Daily and in a news story on KPBS-FM. I deleted the 5-nitroindole fragment from this structure before generating the AutoDock input file for this target. We'll be screening new fragments against this crystal structure's eye site, as well. The Protein Data Bank is located at http://www.rcsb.org/pdb/home/home.do. You can search the PDB using the 4 character codes listed above (i.e., before the .pdb suffix) to learn more about each of these targets. This experiment involves faah11,647 - faah13,984. These calculations began 4/12/2010, they were put "on hold" for a while, and they finished 3/06/2011.
Experiment 31: 100% Completed
Experiment 31 involves screening the Otava library of approximately 335,000 "building blocks" against the "exo sites" on the side surfaces of HIV protease (i.e., the allosteric sites). This experiment will dock these fragments against the exo sites of 8 different targets, which include 5 carefully-selected snapshots from previous Molecular Dynamics simulations of the V82F/I84V multi-drug-resistant mutant "super bug." The other targets correspond to the two new fragment-bound crystal structures of HIV protease that were produced by our collaborator, Prof. C. David Stout at TSRI. We are targeting both sides of one of these two new targets, which is why we have "8 different targets." These two new structures from Prof. Stout were also targeted in Experiments 25-28 and 30. This experiment involves faah8972 - faah11,646. These calculations began 12/07/2009 and ended 4/18/2010.
Experiment 30: 100% Completed
Like Experiment 29, Experiment 30 also uses the "Asinex" library of over 360,000 different compounds in a virtual screen against HIV protease. But in this experiment, the Asinex compounds are being docked against the allosteric inhibitor site (that is, the "exo site" on the sides of HIV protease). In addition, this experiment targets two new fragment-bound crystal structures of protease that were produced by our collaborator, Prof. C. David Stout at TSRI. These two new structures from Prof. Stout were also targeted in Experiments 25-28. This experiment involves faah8234 - faah8971. These calculations began 10/05/2009 and ended 12/07/2009. Update May, 2012: from the initial analysis of the results against the "1F1" allosteric site on the outside/top of the flaps, 10 fragments were purchased (using some of the funds that IBM's "Watson" won on Jeopardy! and which the IBM International Foundation donated to the FightAIDS@Home project). These 10 fragments were analyzed by Max Chang in Prof. Bruce Torbett's lab in "DSF" assays (differential scanning fluorimetry), which measure the thermal stability (melting temperature) of HIV protease in solution. 5 of these 10 fragments caused a significant shift in HIV protease stability in solution, which indicates that these compounds are able to bind to HIV protease. These 5 fragments are now being investigated in further tests performed by the Finn lab, the Elder lab, and the Stout lab.
Experiment 29: 100% Completed
This experiment involves docking a huge library of compounds against the active site of six of our new models of HIV protease (which are a subset of the targets being used in Experiments 25-27). These six targets are the outputs of the equilibration phase of six different Molecular Dynamics simulations (hence the "mdEq" part of the work units' names). The six different types of HIV protease that we are docking compounds against in Exp. 29 include the "Model6Xapo," which is a drug-resistant "super bug" with 6 different mutations. Our collaborator, Prof. Dave Stout, figured out the structure of this 6X mutant. The "apo" part of the name indicates that this mutant protease molecule did not have a substrate or drug present when its structure was solved. This Model6Xapo has semi-open flaps (that is, the two double-arrows that point towards the center of the molecule and form a roof over the active site have opened up). We've been working with the IBM members of the FAAH team to update the graphics on your screen-savers. We've sent them new graphics to use, and they've already started testing them. Soon, you will be able to see exactly what we mean when we say "semi-open flaps." Another new model that is being targeted in Exp. 29 is the wild type HIV protease from 1HHP.pdb. We consider this model to be interesting, because the flaps were fairly open, but then they closed again. But this time, they closed down in the opposite arrangement/they "switched handedness" (that is, the flap that is normally in the front is now in the back). Having this different conformation of the flaps might allow us to fish out new types of interesting compounds for subsequent examination in the "test tube." We included models of a multi-drug-resistant "super bug" with mutations at V82F/I84V and another "super bug" with mutations at I62V/V82A/I84V/L90M. We are targeting a model of the protease molecule from "HIV-1c," as well. HIV-1c is the subtype, or group of strains, that is most commonly found in Asia. We are also targeting a model of "HIV-2" protease with semi-open flaps. HIV2 is the group of strains that are most commonly found in Africa. The current anti-AIDS drugs were developed and optimized against "HIV-1b," which is the subtype most commonly found in Europe and the USA. But some of these anti-HIV protease drugs do not work as well against even the wild type strains that are found in other regions (let alone their "super bugs"). Since we are not controlled by the desire to make profit, we are devoting some of our research efforts to the groups of HIV strains that affect the often-neglected patients in Africa and Asia. In addition, studying these versions of HIV protease can also help us learn how to defeat the "super bugs" we find here in the USA. This experiment is the first one in which we are using the "Asinex" library of over 360,000 different compounds in our virtual screens against HIV protease. This experiment involves faah6022 - faah8233. These calculations began 4/23/2009 and ended 10/20/2009.
Experiment 28: 100% Completed
This experiment utilizes the same set of brand new crystal structures and new models of HIV protease that are being used in Experiments 25-27. See the description of Experiment 25 for the details about these new targets. In experiment 28, we are AutoDocking these compounds against the active site of different variants of HIV protease. This experiment incorporates a library of ligands that we just started using: the ChemBridge building blocks library. The library of "building blocks" from ChemBridge contains many small fragments that were derived from larger compounds. Using these small fragments, or building blocks, should help us cover a larger amount of structural diversity (i.e., of "chemical space") within each experiment. Thus, this library should help us find new hits in a more efficient way. This experiment involves faah5710-6021. These calculations began 4/05/2009 and finished 4/24/2009. Feb. 2011 update: 10 of the top-ranked fragments were purchased and are currently being tested by our collaborators at TSRI. Preliminary data indicate that 2 of these 10 fragments are able to inhibit HIV protease, according to the standard FRET-based activity assay. More testing needs to be done before we can publish these results, but thus far it appears that we discovered 2 completely novel "hits" against the active site/"eye site" in the results of Experiment 28. Extension to Experiment 28 736 compounds from the "ZINC" server that are somewhat similar to these 2 active fragments (discussed above) were hand-picked by Dr. Alex L. Perryman. These 736 compounds generate a "focused library" that we are screening against the active site and the "eye site" of a panel of 9 different variants of HIV protease. Since these 736 compounds are larger than the two active fragments discovered in Experiment 28, this extension of the experiment is searching for more potent compounds that are also able to target both the "eye site" and the floor of the active site. This extension involves faah22,621 - faah22,629. These calculations began 6/01/2011 and ended 6/04/2011.
Experiment 27: 100% Completed
This experiment utilizes the same set of brand new crystal structures and new models of HIV protease that are being used in Experiments 25 and 26. See the description of Experiment 25 for the details about these new targets. In experiment 27, we are targeting the exo site on the sides of HIV protease. This experiment incorporates a library of ligands that we have never used before. We recently downloaded and reformatted the "ChemBridge building blocks library" of ~ 12,000 models of compounds from "ZINC," (which stands for Zinc Is Not Commercial). See the paragraph below for a few details about ZINC. The library of "building blocks" from ChemBridge contains many small fragments that were derived from larger compounds. Using these small fragments, or building blocks, should help us cover a larger amount of structural diversity (i.e., of "chemical space") within each experiment. Thus, this library should help us find new hits in a more efficient way. This experiment involves faah5398 - faah5709. These calculations began 3/26/2009 and finished 4/09/2009.

Kudos to ZINC! The virtual representations of the potential inhibitors that we use in all of these experiments are derived from the libraries of ligands that are freely distributed by "ZINC," (which stands for Zinc Is Not Commercial). ZINC is a free database provided by the Shoichet Laboratory in the Department of Pharmaceutical Chemistry at the University of California, San Francisco (UCSF). To learn more about ZINC, see Irwin, J. and Shoichet, B. J. Chem. Inf. Model. 2005; 45(1):177-82. We thank Dr. John Irwin and Prof. Brian Shoichet for creating and maintaining such a wonderfully useful and free site.
The AutoDock input files ("pdbqt" files) that we generated for several of these ZINC libraries are now available for free at http://zinc.docking.org/pdbqt/.
Experiment 26: 100% Completed
Similar to Experiment 25, this experiment also involves screening the NCI's "DTP library of moderately active compounds" against several brand new structures and new models of HIV protease. But in this experiment, we are docking the potential inhibitors against the active site, instead of the exo site. See the description of Experiment 25 for the details regarding the new structures and models that we are targeting. This experiment involves faah5320 - faah5397. These calculations began 3/16/2009 and ended on 3/31/2009.
Experiment 25: 100% Completed
This experiment involves screening the NCI's "DTP library of moderately active compounds" against the exo site of several brand new structures and new models of HIV-1b protease. (See the description of "Experiment 21" for more details about this DTP library.) This experiment targets the potential allosteric inhibitor site (i.e., the "exo site") on the sides of HIV protease. The new structures utilized in this experiment are based on brand new, currently unpublished x-ray crystallographic structures from our collaborator, Prof. David Stout. The new models of HIV protease that are also included in this experiment were harvested from Molecular Dynamics simulations recently performed by Dr. Alex L. Perryman. We harvested the equilibrated structures from the beginnings of our new MD simulations on several different multi-drug-resistant mutants of HIV-1b protease (i.e., several different "super bugs" against which the current drugs no longer work well.) We also included models of HIV-1c protease ("1c" is the HIV subtype, or group of strains, that are most common in Asia) and HIV-2 protease ("HIV-2" is the predominant subtype in Africa). "HIV-1b" is the subtype of HIV most commonly found in the U.S. and in Europe. The current anti-AIDS drugs were all designed and optimized against HIV-1b, but the FightAIDS@Home project is devoted to trying to help all patients with HIV throughout the world. This experiment involves faah5224 - faah5319. These calculations began on 03/03/2009 and ended on 3/31/2009.
Experiment 24: 100% Completed
Similar to Experiment 12, this experiment performed by Dr. Ruth Huey involves HIV protease "cross-docking" (i.e., this is a test of the new AutoDock code and the new scoring function that involves docking all the known HIV protease inhibitors against 100 different crystal structures of HIV protease). This experiment involved faah4998 - faah5017. These calculations began 08/11/2008 and finished 08/31/2008.
Experiment 23: 100% Completed
Similar to Experiments 19 and 19a, this Relaxed Complex experiment involves docking the different FDA-approved HIV protease inhibitors (and a few compounds still in development) against the active site of 2,000 different snapshots of HIV-1b protease that were harvested from a Molecular Dynamics simulation. However, this experiment involves docking these reference compounds against conformations of the V82F/I84V multi-drug resistant "super bug" of HIV protease. We'll compare the performance of these compounds in this experiment versus their calculated affinities from Experiments 19 and 19a. The reference compounds used in Experiments 19, 19a, and 23 include the FDA-approved drugs amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir, while the compounds in development include AB2, AB3, JE-2147, KNI-272, TL3, and TMC-126. This experiment involves faah4726 - faah4791. These calculations began 12/01/2008, and they finished 02/19/2009.
Experiment 22: 100% Completed
This Relaxed Complex experiment is very similar to Experiment 21, but this time the "DTP" library is being docked against the potential allosteric inhibitor site on the peripheral surface of HIV protease (i.e., the "exo" site), instead of docking them against the active site. These compounds are also being docked against the same "QR-selected" subset of conformations from the V82F/I84V multi-drug-resistant mutant of HIV protease. For a description of the QR method and a few citations, see the description of Experiment 21. Experiment 22 involves faah4417 - faah4622 and faah5018 - faah5223. These calculations began 10/18/2008, and they ended 03/05/2009.
Experiment 21: 100% Completed
This Relaxed Complex experiment is testing both a new library of ligands and a new method for selecting the snapshots of the target from MD against which to dock these compounds. The first 1/3 of ligands from the NCI's "DTP" library of compounds is being tested now, while we prepare the files that describe the other 2/3 of this library of compounds. **Update 07/31/2008** All 3/3 of this library have now been prepared by Dr. Stefano Forli. The other 2/3 of this experiment have been submitted. These compounds were "moderately active" in cell-based assays at the NCI, but noone knows which targets these bind to or how they are able to inhibit them. These compounds are being docked against the active site of a "QR-selected" subset of conformations harvested from Molecular Dynamics simulations of the V82F/I84V multi-drug-resistant mutant of HIV protease (i.e., a target from one of the most drug-resistant "super bugs" of HIV). The Structure QR method is a new tool for selecting a structurally diverse, non-redundant set of conformations from a group of different structures that have similar sequences. We thank John Eargle of the Luthey-Schulten lab at UIUC for helping us learn how to apply this method. For more info. on QR, see P. O'Donoghue and Z. Luthey-Schulten; Evolutionary profiles derived from the QR factorization of multiple structural alignments gives an economy of information; J. Mol. Biol., 346, 875-894, (2005). See also MultiSeq of VMD: Elijah Roberts, John Eargle, Dan Wright, and Zaida Luthey-Schulten; MultiSeq: Unifying sequence and structure data for evolutionary analysis; BMC Bioinformatics, 7:382 (2006). This experiment involves faah4314 - faah4416, faah4623 - faah4725, and faah4792 - faah4997. These calculations began 08/28/2008, and they finished 2/19/2009.
Experiment 20: 100% Completed
This Relaxed Complex experiment is similar to Experiment 16, but different run parameters are being used during the docking and several different protocols for preparing the input files of these compounds are being tested (such as using different protocols to calculate the charges on the atoms within each ligand, using different "atom types" to describe the ligands, and using different protocols for minimizing the structures of the ligands). Thus, this experiment will also allow us to investigate the best way(s) for preparing ligands that will be used in subsequent Relaxed Complex experiments. This experiment involves faah4202 - faah4313. These calculations began 06/08/2008, and they ended on 9/30/2008.
Experiment 19a: 100% Completed
This Relaxed Complex experiment involves docking the different FDA-approved HIV protease inhibitors (and a few compounds still in development) against the active site of 2,000 different snapshots of the wild type HIV-1b protease that were harvested from the same Molecular Dynamics simulation discussed above in Exp. 19. These calculations will provide a base-line against which to compare the performance of the compounds used in Experiment 19. Different protocols for preparing the input files of these current drugs were used (such as using different protocols to calculate the charges on the atoms within each ligand, using different "atom types" to describe the ligands, and using different protocols for minimizing the structures of the ligands). Thus, this experiment will also allow us to investigate the best way(s) for preparing ligands that will be used in subsequent Relaxed Complex experiments. The reference compounds used in Experiment 19a include the FDA-approved drugs amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir, while the compounds in development include AB2, AB3, JE-2147, KNI-272, TL3, and TMC-126. This experiment involved faah4070 - faah4201. These calculations began 05/26/2008 and finished 08/19/2008.
Experiment 19: 100% Completed
This experiment is similar to Exp. 13, but different run parameters are being used (for example, 2 point cross-over is being used, while Exp. 13 used the arithmetic crossover protocol in the genetic algorithm used in the docking calculations; a new and improved version of the AutoDock code is being used, as well). Exp. 19 is a Relaxed Complex experiment of the "9 false negatives" from the NCI Diversity Set. These 9 compounds did not dock well in previous experiments (by Max Chang and Dr. Lindy Lindstrom) that targeted different crystal structures of HIV protease, but they did display some activity in an experimental assay against HIV protease. Different versions of these 9 ligands and of a few reference compounds are being docked against the active site in 2,000 different snapshots of the wild type protease that were harvested from Dr. Alex Perryman's previously-published Molecular Dynamics simulations (i.e., the cover article of the April, 2004, issue of Protein Science). The reference compounds used in Experiment 19 include the FDA-approved drugs amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir, while the compounds in development include AB2, AB3, JE-2147, KNI-272, TL3, and TMC-126. To view some of the recent results from the Relaxed Complex experiments on these reference compounds, see the graph with part of Indinavir's Relaxed Complex "trajectory" or the comparison of AB2's versus AB3's RC "trajectories" at the bottom of this page. This experiment involved faah4000 - faah4069. These calculations began 05/02/2008 and finished 06/14/2008.
Status 1-18
Experiment 18: 55% Completed--Has Been Halted
NCI Diversity Set (1900) vs. 500 conformations from the first 5 ns of MD on the V82F/I84V drug-resistant "super-bug" of HIV protease. These results will be compared to those from Experiments 10, 11, and a future experiment, in order to help test and improve the methods and tools that can be used in drug design research against any target of interest. This experiment involves faah3202-3702. This experiment is now "on hold." It was halted before its completion, because an error was discovered in the code. The massive number of calculations that you perform for us helped us discover a serious error, and now we have made it much harder to accidentally mis-compile the code. In addition, we have now created new methods for checking the quality of the results. Thus, by helping us improve our code and our protocols, this little setback will help our research in the long-run, and it will help the research that over 4,000 other labs perform with the AutoDock code. We will repeat this experiment (or something very similar to it) with the WCG's new version of the AutoDock code, which is now up-and-running.
Experiment 17: 88% Completed--Has Been Halted
Relaxed Complex Method of Several Compounds, Their Fragments & Several Derivatives versus the Exo site of snapshots from MD of the V82F/I84V drug-resistant "super-bug" of HIV protease (from 1D4S.pdb). This experiment involves faah3145-3201. This experiment is now "on hold." It was halted before its completion, because an error was discovered in the code. The massive number of calculations that you perform for us helped us discover a serious error, and now we have made it much harder to accidentally mis-compile the code. In addition, we have now created new methods for checking the quality of the results. Thus, by helping us improve our code and our protocols, this little setback will help our research in the long-run, and it will help the research that over 4,000 other labs perform with the AutoDock code. We will repeat this experiment (or something very similar to it) with the WCG's new version of the AutoDock code, which is now up-and-running.
Experiment 16: 100% Completed
Relaxed Complex Method of Several Compounds, Their Fragments & Several Derivatives versus the Exo site of snapshots from MD of Wild Type 1KZK
Experiment 15: 100% completed
Relaxed Complex Method of the NCI Diversity Set versus the Exo site of snapshots from MD of Mutant 1D4S
Experiment 14: 100% Completed
Relaxed Complex Method of the NCI Diversity Set versus the Exo site of snapshots from MD of Wild Type 1KZK
Experiment 13: 100% Completed
Relaxed Complex Method of the 9 false negatives from the full NCI Diversity Set
Experiment 12: 100% completed
HIV protease cross-docking (i.e., this is a test of the new AutoDock code and the new scoring function). This experiment helps us test and improve the tools that we use in these calculations (and that thousands of other labs use in their drug design research against other diseases). This experiment involves faah2695-2714.
Experiment 11: 100% Completed
NCI Diversity Set (1900) vs. 500 conformations from the first 5 ns of MD on wild type HIV protease
Experiment 10: 100% Completed
This was Dr. Alex Perryman's first experiment on FAAH. The NCI Diversity Set of compounds was docked against 7 "interesting" snapshots of the V82F/I84V multi-drug-resistant "super bug" of HIV protease. These snapshots were deemed "interesting," since they seemed to be very useful in Dr. Perryman's previous Relaxed Complex experiments. That is, these compounds displayed some of the lowest (i.e., best) Free Energies of Binding against the inhibitor JE-2147, and these 7 conformations helped resolve the compounds that bound well from those that did not bind (in previous computational experiments).
Experiment 9: 100% Completed
The "consensus" wild type crystal (2BPW.pdb) was used to screen Max Chang's version of the "DTP library of moderately-active compounds" from the NCI. For details on why this wild type protease is considered a "consensus wt," follow the link on the previous page to the FAAH paper published in the Journal of Chemical Information and Modelling.
Experiment 8: 100% Completed
Experiment 8b: 100% Completed Drugs et al. vs. SCWRL-modeled mutants (no H2O) with flexible sidechains Experiment 8a: 100% Completed Drugs et al. vs. SCWRL-modeled mutants (no H2O) (rigid)
Experiment 7: 100% Completed
Experiment 7b2: 100% Completed x2AZ8 vs ZINC Fragment Library (exo site) Experiment 7b1: 100% Completed x2AZ8 vs ZINC Fragment Library (active site) Experiment 7a2: 100% Completed x2BPW vs ZINC Fragment Library (exo site) Experiment 7a1.1: 100% Completed Fragment Library vs 2BPW (Active Site)
Experiment 6a, 6b, 6c, 6d: 100% Completed
NCI vs. 1MEU, 2BPZ, 2BPW, & 2AZ8
Experiment 5: 100% completed
BindingDB vs. HIV Protease, with and without Active Site Water Molecule
Experiment 4: 100% Completed
NCI Diversity Set vs. HIV PR Monomer
Experiment 3: 100% completed
Testing Sidechain Motion in HIV Protease Cross Dockings
Experiment 2: 100% Completed
ChemBridge (500,000) vs. Wild Type HIV Protease (1) Top Hits from Stage 1 vs. Mutant HIV Protease Panel (270) NCI Diversity Set (1,900) vs. Monomeric HIV Protease (20)
Experiment 1: 100% completed
Experiment 1b: 100% completed NCI Set (230,000) vs. Wild Type HIV Protease (1) Experiment 1a: 100% Completed NCI Diversity Set (1,900) vs. Mutant HIV Protease Panel (270)