OUR RESEARCH
Reaction Progress Kinetic Analysis
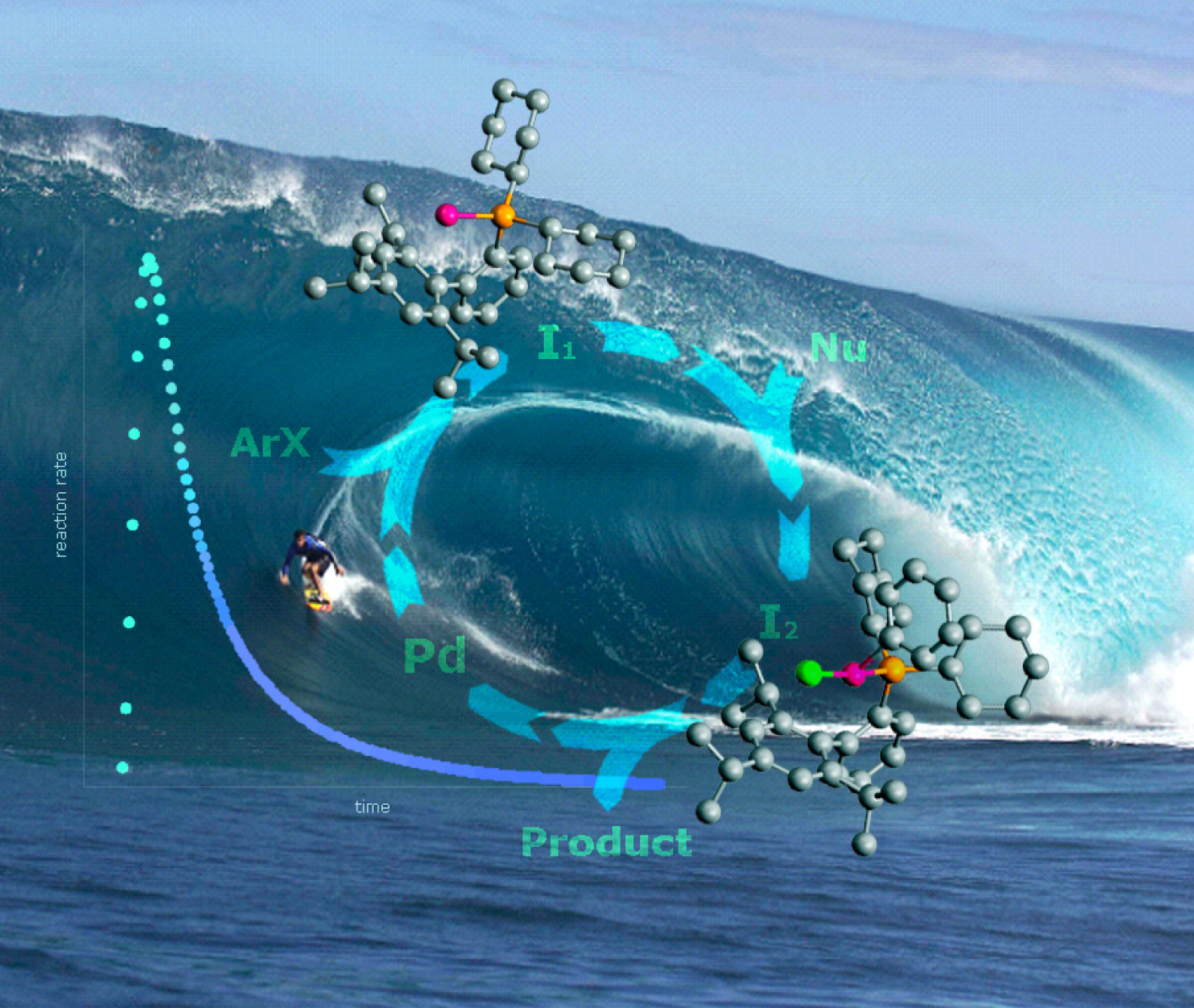
In situ monitoring of reaction progress using modern experimental tools such as reaction calorimetry allows us to "ride" a complex catalytic reaction cycle much as a surfer rides along the tube of a breaking wave.
Following the global kinetics as the catalytic intermediates in a cycle enable the passage of reactants through to products provides a wealth of information that aids in the design and interpretation of further mechanistic experiments. Prof. Blackmond has pioneered the development of Reaction Progress Kinetic Analysis (RPKA), a methodology combining highly accurate in-situ data collection with a rigorous mathematical analysis that permits rapid determination of concentration dependences of reactants. Reaction Progress Kinetic Analysis offers a visual, graphical approach in which a critical minimum set of carefully designed experiments permits rapid extraction of kinetic information even as several concentration variables are changing at once. One of the most powerful aspects of the methodology is its ability to deconvolute rate processes occurring on the catalytic cycle from those occurring off the cycle. Reaction Progress Kinetic Analysis finds important application in the pharmaceutical industry, where streamlining process R&D based on Blackmond’s kinetic analysis is becoming an industry-wide standard.
RPKA represents a temporal scan of reactant and product concentrations over the course of the reaction, turning concentration from a discrete variable into a virtually continuous one. In an expansion of this approach, the Blackmond lab developed the Temperature Scanning Reaction (TSR) protocol, which employs a temporal temperature ramp to screen concentrations and temperature over the course of the reaction. Using reaction calorimetry, this methodology provides both kinetic and thermodynamic information about reaction progress.
Mechanistic Investigations of Catalytic Reactions
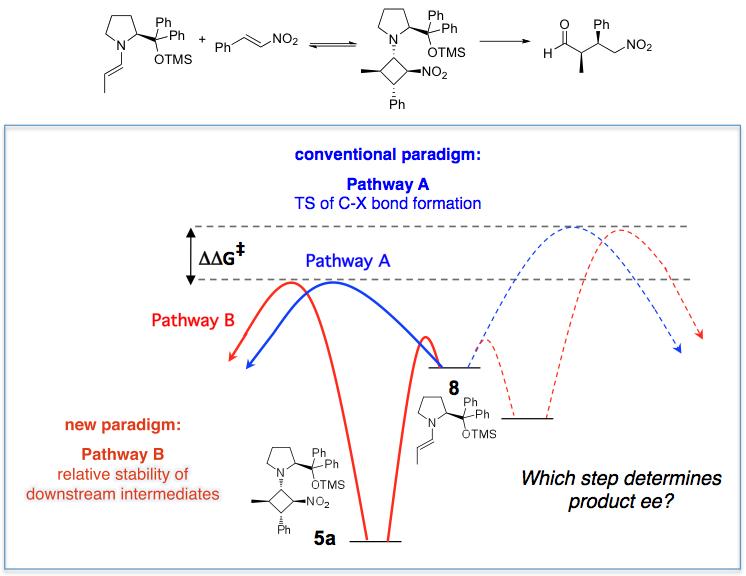
Catalytic reactions are dynamic systems encompassing multiple sequential and concurrent steps; an asymmetric catalytic reaction has, by definition, at least two reaction pathways. Temporal monitoring of these reactions, combined with other spectroscopic and structural tools, can provide a wealth of information far beyond simple yield and selectivity measurements. To use paleontology as an analogy, we might say that basing a mechanistic model on comparison of endpoint yield analyses of complex catalytic reactions is akin to describing the characteristics of a dinosaur by studying its bones. While paleontologists do not have the luxury of being able to observe their dinosaurs while they are running, chemists do have the opportunity to monitor the dynamic behavior of reactions.
In contrast to the classical role of kinetics, in which measurements of concentration dependences most often are asked simply to corroborate a previously proposed mechanism, the Blackmond group’s approach is to employ kinetic studies at the outset of an investigation of ill-defined reaction network to suggest reaction mechanisms. This “kinetic-assisted mechanistic analysis” aids in the design of further supporting experiments including conventional mechanistic tools such as studies of isotope effects and spectroscopic studies for structural and compositional information. Prominent examples of our mechanistic studies of complex organic reactions and reaction networks include asymmetric hydrogenation, asymmetric organocatalytic reactions, Pd-catalyzed C-C and C-N bond forming reactions, and transition-metal catalyzed competitive reactions including kinetic resolutions. Most recently, we have turned our attention to complex, multi-cycle reaction networks found in free radical reactions, photocatalysis, and electrocatalysis. We have introduced the term “kinetic matching” to describe how we use kinetic investigations in these networks that operate as a cascade or multi-cog system to optimize reaction conditions.
Nonlinear effects of catalyst enantiopurity
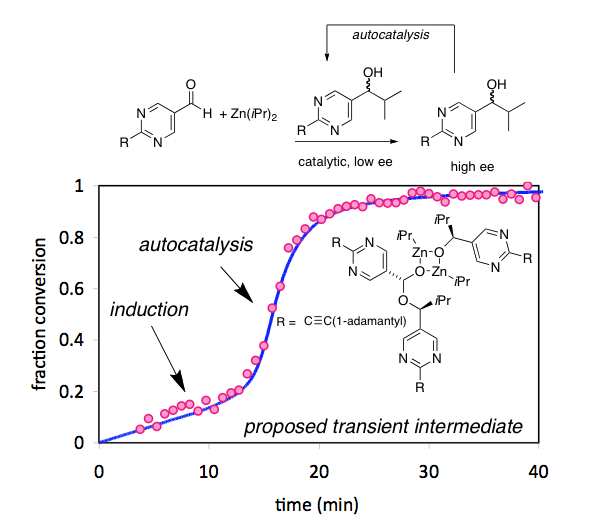
Experimental and theoretical studies in the Blackmond group have derived relationships between catalyst ee and reaction rate that complement the standard tool of studying product ee as a function of catalyst ee. This work provides a means of testing proposed models for nonlinear effects and expands the power of studies of nonlinear effects to serve as a meaningful mechanistic probe, including nonlinear effects that arise from complex network behavior, from phase behavior considerations in addition to simple studies of higher order catalyst species both on and off the catalytic cycle.
Biological homochirality and amino acid phase behavior
The single chirality of the amino acids and sugars that make up the building blocks of life has fascinated scientists and laymen alike since Pasteur’s first painstaking separation of the enantiomorphic crystals of a tartrate salt over 150 years ago. Autocatalytic self-replication has been highlighted as a potential route to rationalize how one enantiomer might have come to dominate over the other from what presumably was a racemic prebiotic world. The Blackmond group recently demonstrated how two reactions, the dipeptide-catalyzed transamination to produce enantioenriched amino acids, and the thiol-catalyzed ligation of amino acids to produce dipeptides, might be combined to produce a prebiotically plausible network for chiral amplification.
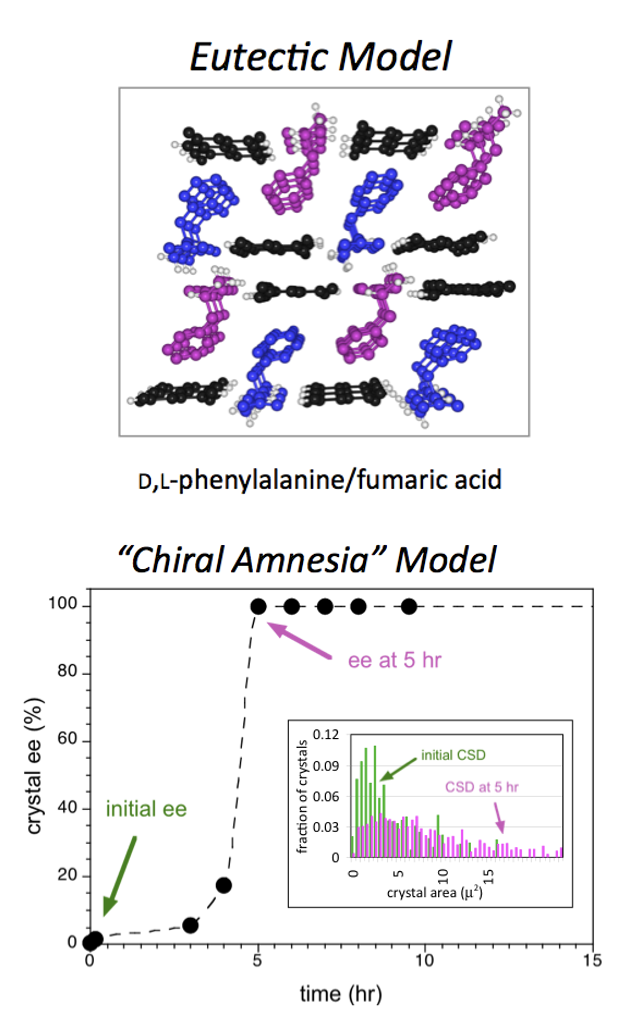
The Blackmond group has expanded the range of models to rationalize the origin of biological homochirality from proposals based purely on chemical reactions to those based on physical phase behavior of chiral molecules as well as a combination of chemical and physical processes. Highly enantioenriched solutions of amino acids can be produced from nearly racemic mixtures via solution-solid partitioning of the enantiomers.
Reactions catalyzed by amino acids that are carried out in such systems show nonlinear product ee consistent with this highly enantioenriched solution composition. This concept was then greatly expanded in scope with the discovery that eutectic compositions could be “tuned” by judicious choice of additives that alter crystal structure and solubility. In sharp contrast to autocatalytic reaction models, which invoke “far-from-equilibrium” behavior, this eutectic model is a pure equilibrium treatment. This distinction has important implications for scenarios concerning the time course over which the evolution of homochirality may have developed. Probing the phase behavior of amino acids in conjunction with solution racemization led to separate work showing how one hand of a chiral solid could be transformed completely into its enantiomorph from a nearly racemic mixture of the two. Because interconversion in solution allows an enantiomer that had been part of an L crystal to become part of a D crystal, this has been dubbed the “chiral amnesia” process.