Research
"No one is so old as to think that they cannot live one more year.” Cicero
Old age is accompanied by deterioration of tissues and organs, increased susceptibility to disease, and an exponential increase in mortality. The last years have seen great progress in unraveling the mechanisms of aging. It has become clear that aging is modulated by various genetic pathways which when properly manipulated can lead to an increase in lifespan. These findings have raised the exciting possibility that onset, progress or outcome of age related diseases in humans could be slowed by drug targeting aging instead of the disease itself. To develop small molecule therapeutics that target aging we have identified three key areas that require scientific progress.- Mechanistic understanding on how small molecules slow aging and extend lifespan.
- Metrics to determine the efficacy of a lifespan-extending drug prior to death.
- To understand the molecular mechanisms by which aging drives age-related disease such as Alzheimer’s or Parkinson’s.
To investigate these questions we have screened large libraries for small molecules that extend lifespan in C. elegans, a short-lived model organism. We have identified over 100 lifespan-extending molecules and have generated a pharmacological network of lifespan extension that forms the basis of our research.
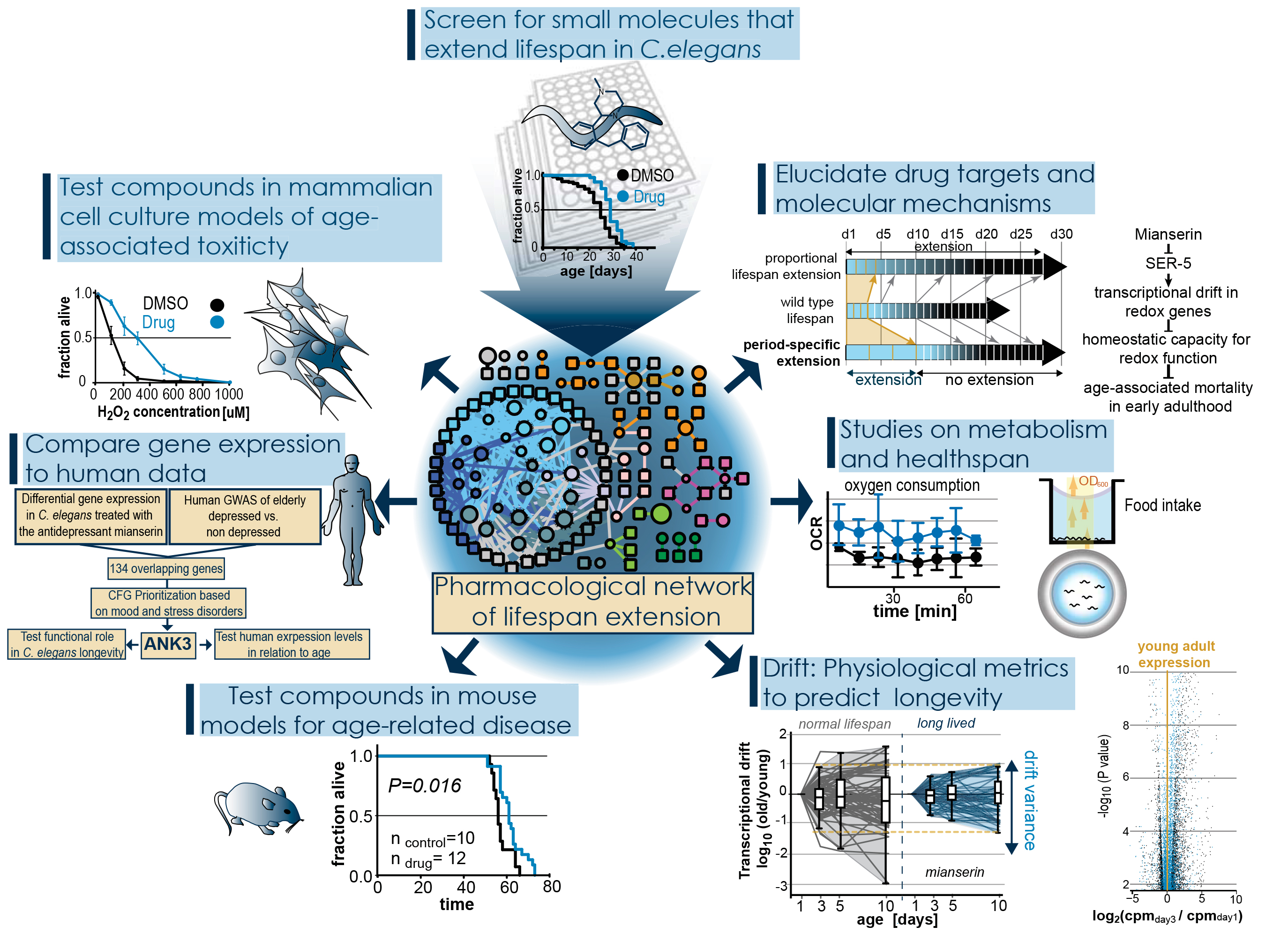 |
Mechanistic Understanding
To identify which molecules act by evolutionarily conserved mechanisms we are testing the lifespan-extending compounds identified in C. elegans in mammalian cell culture models of age-associated toxicities such as proteotoxic stress, oxidative stress or ischemia. Many of the compounds we identified have been FDA approved allowing us further to compare available human data with our C. elegans data. To finally understand the mechanisms by which these molecules extend lifespan and delay aging we are using chemical, genetic and biochemical techniques to identify their targets and thus the mechanism by which they extend lifespan.
Metrics of Aging
While in model organisms it is possible to determine whether a molecule slows aging by measuring lifespan, this is not possible in long-lived species. Thus our lab is developing methods to measure biological aging. These include studies on health span involving movement, stress resistance, food intake and oxygen consumption. We further have discovered a phenomenon we call transcriptional drift. Transcriptional drift describes the age-associated loss of stoichiometric balance within pathways or transcriptomes and we believe that this progressive loss of stoichiometry drives the age-associated loss of pathway capacities as well as functional decline. Drift allows us to observe the aging process at the molecular level on the basis of transcriptomes, proteomes or metabolomes and to identify lifespan-extending interventions long before the animals die.
Aging and Neurodegenerative Disease
Using the large number of lifespan-extending small molecules as tools allows us to study the relationship of aging and age-related disease. By treating different C. elegans or mouse models of age-related disease, it allows us to ask if all aging-pathways are equally well suited to target a specific age-related disease or not, and which aging mechanisms are optimal to treat which diseases.
Principal Investigator
Michael Petrascheck
Associate Professor
Ph.D. University of Zurich
Phone: (858) 784-8095
e-mail: pscheck@scripps.edu