|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Self-assembling peptide nanotubes
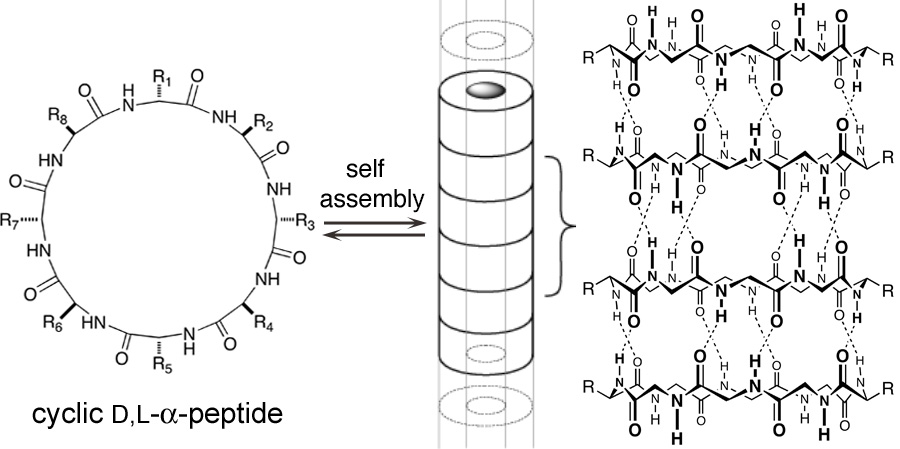
Nanomedicine holds great promise for revolutionizing the treatment of disease, but constructing molecular devices that functionally modulate nanometer-scale biological complexes, such as most protein complexes, remains a challenge. Our lab was the first to show experimentally that cyclic peptides with an even number of alternating D- and L-alpha-amino acids adopt flat, ring-shaped conformations that can stack to form hollow, beta-sheet-like tubular structures with the amino acid side chains on the outside surface of the nanotube. The self-assembly is driven by hydrogen bonding between the backbone amide groups, which are oriented perpendicular to the side chains and the plane of the ring. Importantly, the self-assembly is reversible, such that a highly dynamic assembly process takes place. We have shown that certain six- and eight-residue cyclic D,L-alpha-peptides act preferentially on Gram-positive and/or Gram-negative bacterial membranes compared to mammalian cells, increase membrane permeability, collapse transmembrane ion potentials, and cause rapid bacterial cell death. The effectiveness of this class of materials as selective antibacterial agents is highlighted by the high efficacy observed against lethal methicillin-resistant Staphylococcus aureus (MRSA) infections in mice. We have also found that self-assembling cyclic D,L-alpha-peptides hold considerable potential as a new supramolecular approach toward the design and discovery of broad-spectrum antiviral agents. We have discovered peptide sequences that are effective in preventing infections by HCV and adenovirus. We are continuing to study the cyclic peptdes for a number of therapeutic applications.
|
|||||||||||
| Copyright 2019 © Ghadiri Laboratory, The Scripps Research Institute |