
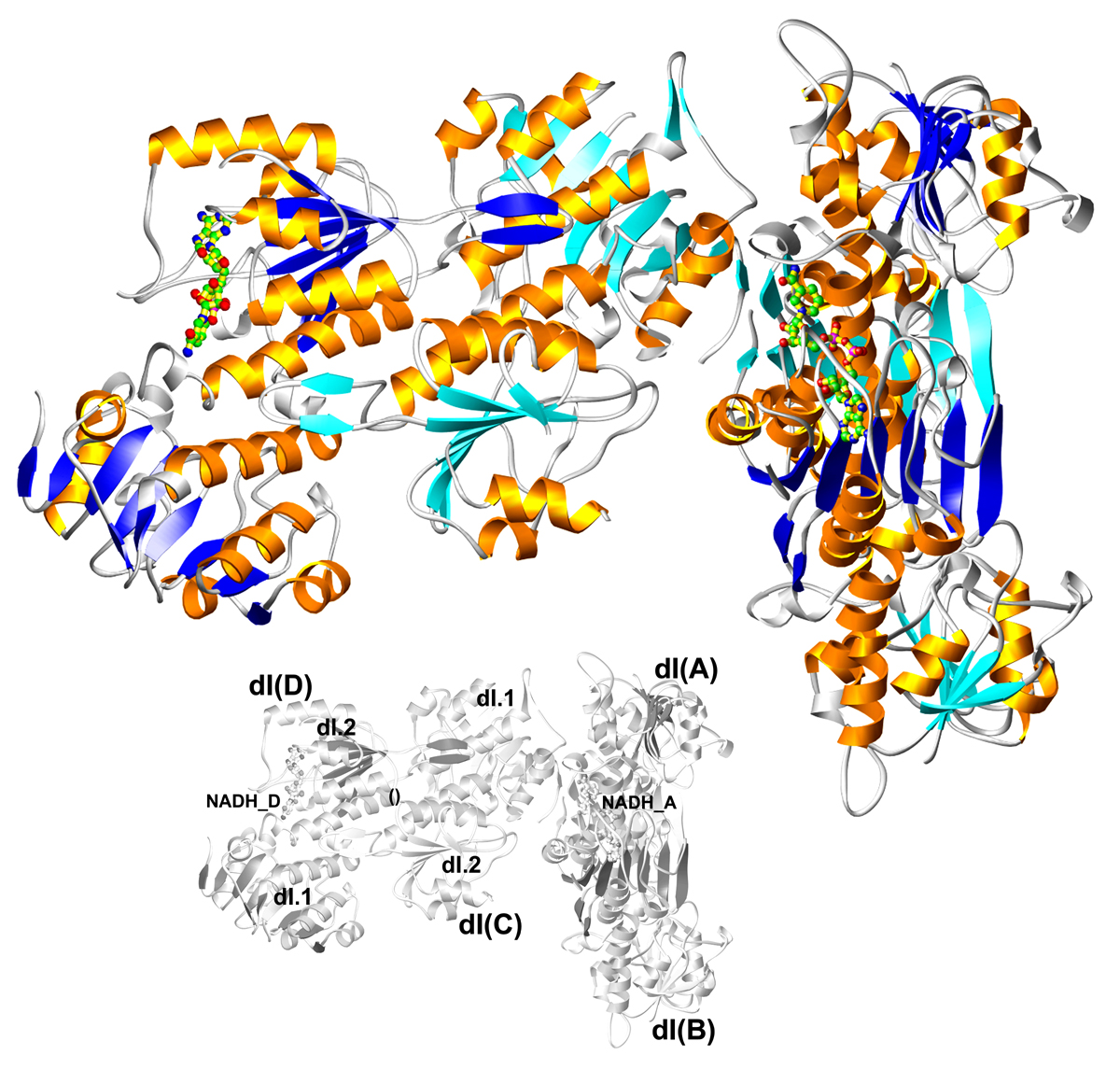
The mitochondrial enzyme transhydrogenase transports protons across the membrane in concert with hydride exchange between NAD(H) and NADP(H), a reaction that couples the proton motive force to the concentration of reducing equivalents in mitochondria. In collaboration with Y. Hatefi, structures of the soluble NADP(H) and NAD(H) binding domains have been determined, while the key residues involved in proton translocation through the integral membrane domain have been defined in biochemical studies. Crystal structures of the NAD(H) binding domain in both oxidized and reduced states have revealed conformational changes due to the presence of NAD vs. NADH; structure solution of the oxidized and reduced states of the NADP(H) binding domain is in progress. The combined results from the crystal structures, and extensive mutagenesis and biochemical assays, are defining the mechanism by which transhydrogenase utilizes cofactor binding energy for the proton translocation.
Home
