Chemical glycobiology
Our group investigates the roles of glycan binding proteins that mediate cellular processes central to immune regulation and human disease. We work at the interface of biology and chemistry to understand how the interaction of glycan binding proteins with their ligands mediates cell-cell interactions, endocytosis and cell signaling. Our multi-disciplinary approach is complemented by a diverse group of chemists, biochemists, cell biologists, and molecular biologists.
Biological roles of siglecs.
The siglecs are a family of fourteen sialic acid binding proteins that function as cell signaling co-receptors and are primarily expressed on various leukocytes that mediate acquired and innate immune functions including B cells, eosinophils, macrophages, dendritic cells and NK cells. Siglecs are a subfamily of the immunoglobulin superfamily that have a unique N-terminal Ig domain that binds sialic acid containing carbohydrate groups (sialosides) of glycoproteins and glycolipids as ligands. The cytoplasmic domains of most siglecs contain tyrosine-based motifs that regulate signaling of cell surface receptor proteins.
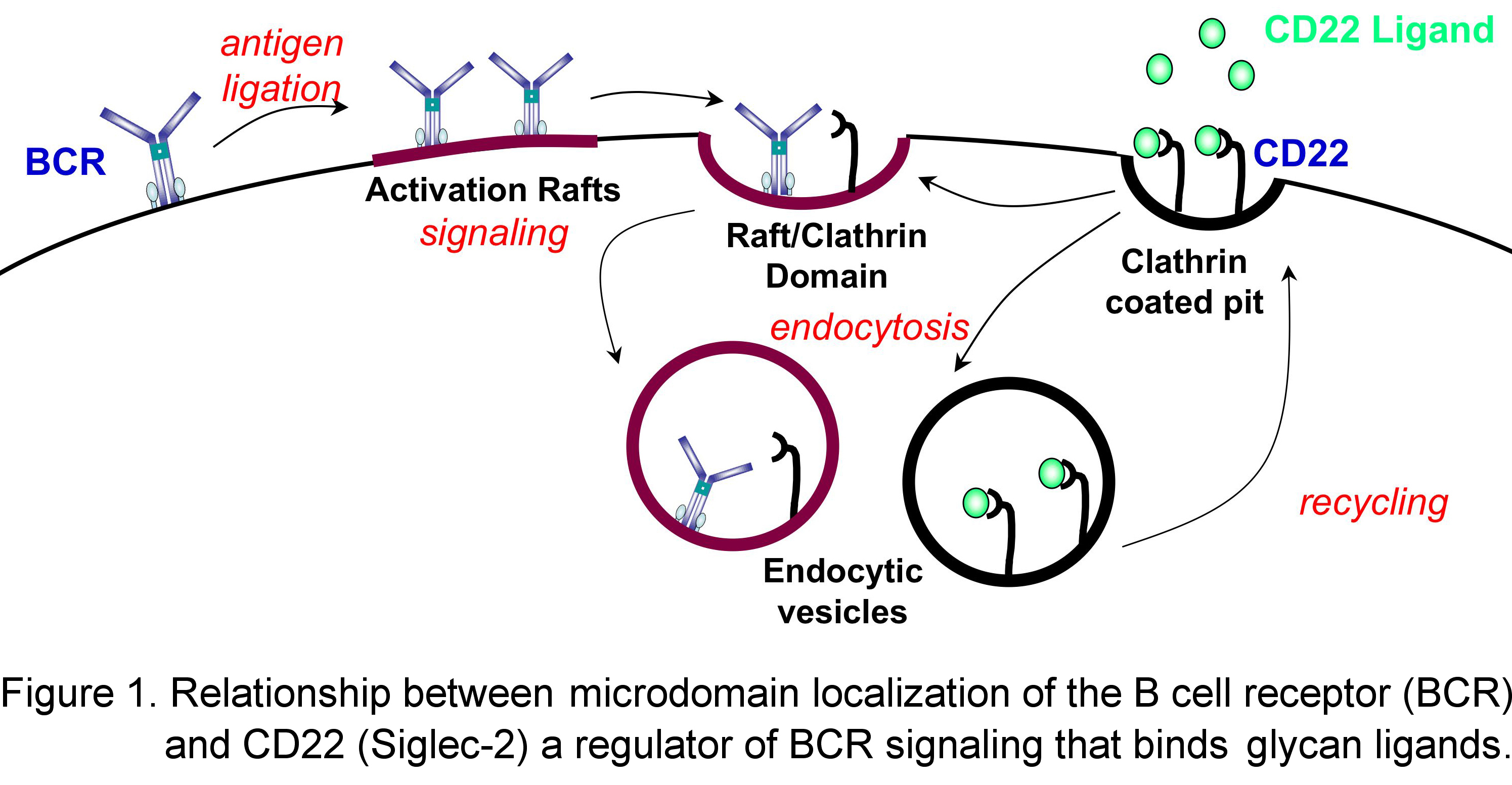
Exemplary is CD22 (Siglec-2), an accessory molecule of the B-cell receptor complex (BCR) that exhibits both positive and negative effects on receptor signaling. The carbohydrate ligand recognized by CD22 is the sequence Siaa2-6Galb1-4GlcNAc found on both neighboring glycoproteins of the same B cell (cis ligands) ) and on cells that interact with B cells (e.g. T cells, trans ligands).
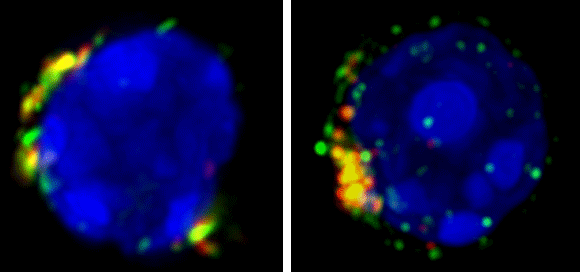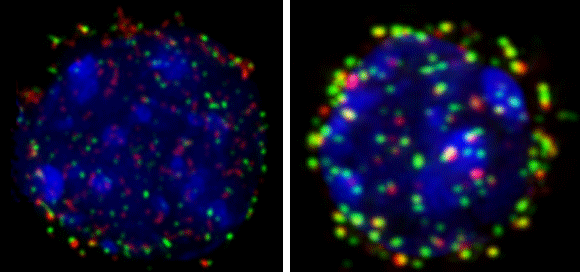
Interactions of CD22 with cis or trans ligands regulate aspects of B cell activation, proliferation and development. Following antigen activation in wild type mice, the BCR is endocytosed via raft-clathrin domains, which we believe is the site of CD22 regulation of BCR signaling. In resting cells CD22 undergoes constitutive endocytosis, resulting in rapid internalization of high affinity glycan ligands or anti-CD22 antibody (Figure 1).
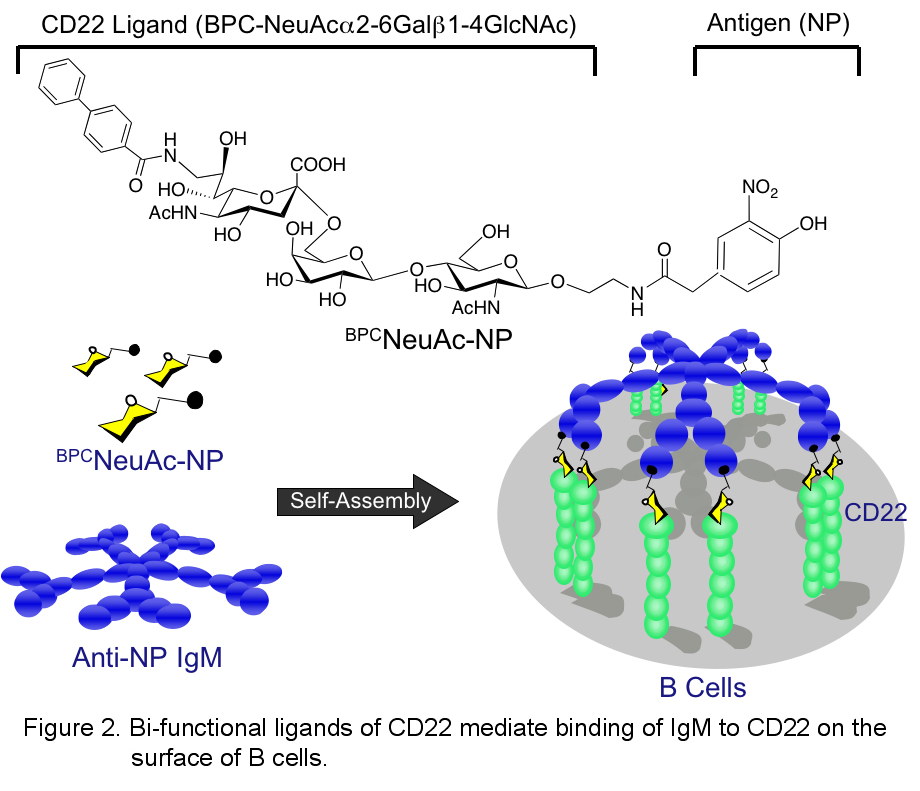
In recent years we have successfully designed robust multivalent platforms displaying glycan ligands that compete with endogenous (cis) ) ligands and bind to native siglec expressing cells. For example, we have found that bi-functional molecules comprising a high affinity ligand of CD22 (BPCNeuAc) coupled to an antigen (NP) will dock an anti-NP antibody (IgM, IgA or IgG) to CD22 on the surface of B cells. In effect, the antibody serves as a multivalent protein scaffold that promotes spontaneous assembly of an immune complex on the surface of B cells driven by the bi-functional ligand of CD22 (Figure 2).
Because the bound ligands are rapidly internalized, they can be used to carry cargo into the cells. Recently we have found that high affinity glycan ligands of CD22 coupled to a pegylated lipid allows formation of liposomes that bind and are endocytosed by B lymphoma cells. When loaded with the chemotherapeutic doxorubicin, these liposomes target human B cells and prolong survival in a murine model of human B cell lymphoma.
Current efforts are directed to developing high affinity ligands for siglecs expressed on other cells, including macrophages, eosinophils and dendritic cells, which will expand the armamentarium for understanding the biological roles of the siglecs and selectively targeting siglec expressing cells in vivo.
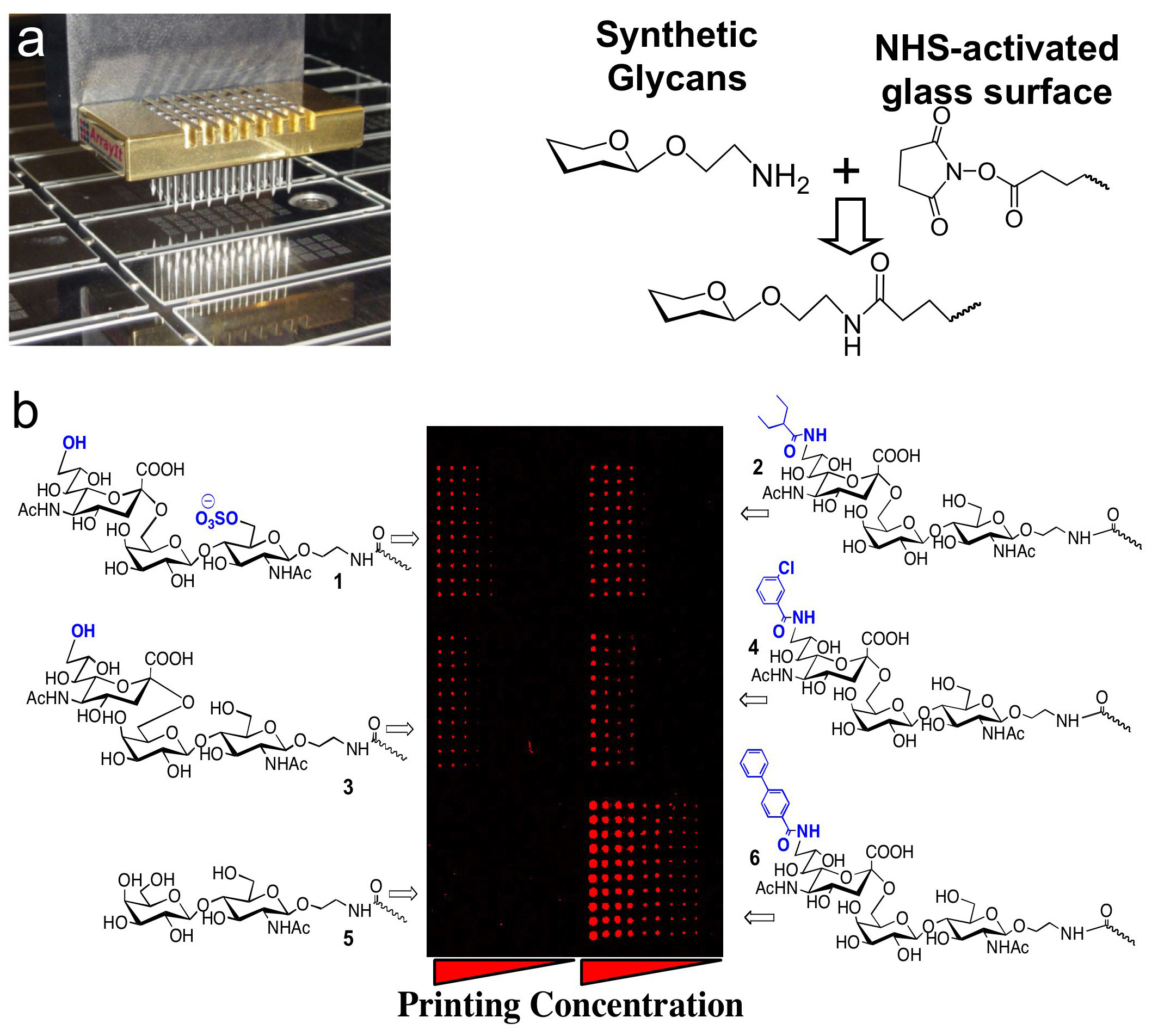
Sialoside analog glycan arrays.
To aid development of ligands for the siglec family, we have embarked on a major effort to identify high affinity ligand analogs of each siglec. To this end we have developed a robotically printed glycan array displaying sialoside analogs to assess the affinity of siglecs for unnatural substituents at the C-9 and C-5 positions of sialic acids (see also Consortium for Functional Glycomics below). Experiments to date using arrays of up to 220 substituents at the C-9 and C-5 positions of sialic acid, have proven to be a powerful method for identifying substituents that increase the affinity of the natural ligand for siglecs by 100-fold or more (see example in Figure 3).
Results from the array can be rapidly assimilated into the synthesis of high affinity ligands and ligand-based probes of the corresponding siglec using flexible chemo-enzymatic synthesis strategies.
Our group also produced a library of synthetic glycans by chemo-enzymatic synthesis used for production of the glycan microarray of the Consortium for Functional Glycomics (CFG) by the TSRI Microarray. This array today contains over 600 glycan structures and is used for analysis of the specificity of glycan binding proteins submitted by CFG investigators from around the world. This array and related custom sialoside arrays have been used in collaborations with the laboratory of Ian Wilson and the Centers for Disease Control to investigate the molecular basis of the specificities of the 1918 pandemic influenza, and emerging avian influenza viruses (e.g. H5N1) to identify mutations required to switch specificity from avian receptors (NeuAca2-3Gal) to human type receptors (NeuAca2-6Gal).
KEY WORDS:
Carbohydrate; Siglec; CD22; lymphocyte; signaling; sialic acid; glycan; macrophage; dendritic cell
|

|
|