Scientists Clone Protein That Fights Bacteria and Viruses
By Jason Socrates
Bardi
Bacteria and viruses are completely different classes of
pathogens, and not surprisingly the body uses completely different
molecular "receptors" to detect them in order to mount an
immune defense.
Paradoxically, while the detection systems are different,
the actual immune defenses the body employs to clear the system
of viral or bacterial infection are much the same. As are
the symptoms—to you or me, fighting off bacteria or viruses
can produce the same fatigue, inflammation, or hacking cough.
Now a team of researchers at The Scripps Research Institute
(TSRI) has published a paper appearing in an upcoming issue
of the journal Nature that explains how pathogens as
different as viruses and bacteria can have such a common bottom
line.
"The proximal reason [for these similar symptoms] is a single
protein," says TSRI Professor Bruce Beutler, who led the research.
This protein, called Trif, associates with different "receptors"
that detect a virus or a bacterium on the surfaces of human
cells. Trif is a signal transducer—it helps turn these
positive detections into immune reactions. Significantly,
Trif is the topmost protein shared by the pathway that detects
gram-negative bacteria and the pathway that detects most viruses.
It is like a waiter who brings orders from two different customers
into the same kitchen.
This is the first time that anyone has identified a protein
that directly responds to the signals the innate immune system
sends when it recognizes both bacteria and viruses.
In addition, Trif could be a potential target for intervening
in diseases in which the innate immune system plays a role,
such as sepsis. Sepsis basically results from a runaway cascade
of inflammation in response to a bacterial infection, and
Trif is involved very early in this cascade. If drugs might
be designed that could modulate the function of Trif, they
might help to improve the prognosis for sepsis.
"You could imagine that blocking this pathway would have
a pretty strong anti-inflammatory effect in a diverse range
of infectious diseases," says Beutler, who identified and
cloned the Trif gene (called Lps2) together with Kasper
Hoebe, Ph.D., a postdoctoral fellow in the Beutler laboratory.
TSRI Associate Professor Jiahuai Han, Ph.D. and Sung Kim,
Ph.D., a postdoctoral fellow in Han's laboratory, collaborated
in this effort.
Mapping the Gene
Beutler, Hoebe, and their colleagues mapped the mouse gene
Lps2, which has an equivalent gene in humans, after
they found a deleterious mutation in a mouse gene that made
mouse macrophages unable to sense certain pathogens, thus
weakening their innate immune systems.
"Mice that lack this protein are very susceptible to infections
like mouse cytomegalovirus," says Beutler, adding that the
mice are also unable to respond to bacterial endotoxins, like
lipopolysaccharide (LPS) molecules, which are found in the
cell walls of many bacteria. In mammals, the innate immune
system detects LPS and a multiplicity of other foreign molecules
with a family of receptors called the toll-like receptors
(TLRs). Mammals have 10 or more different TLR receptors, and
one of the goals of scientists like Beutler is to identify
how these receptors mediate innate immunity.
Innate immunity is essential for survival in a world filled
with microbial pathogens because cells of the innate immune
system are the body's first responders, arriving soon after
foreign pathogens are detected.
Normally, when human or mouse cells encounter bacteria or
viruses, they recognize them with the help of TLRs and other
proteins such as Trif. This recognition triggers the immune
system, which responds with a multi-stage biochemical defense.
The first stage typically involves the innate immune system
and its army of white blood cells, like macrophages, which
engulf and destroy pathogens. The macrophages also fight the
pathogens by producing chemicals at the site of an infection
that induce inflammation. One of these chemicals is called
tumor necrosis factor alpha (TNF-alpha). Normally, TNF-alpha
is produced in great amounts by macrophages when they are
exposed to bacterial and viral "ligands"—the molecules
found in the cell walls of bacteria, for instance.
Beutler and his colleagues were able to identify the function
of Trif and clone the Lps2 gene after they first observed
how a random mutation in one mouse rendered its macrophages
unable to produce TNF-alpha when exposed to LPS from gram-negative
bacteria like E. coli or when exposed to double-stranded RNA—a
product of many viral infections. LPS is known to signal via
TLR4: a discovery made by TSRI investigators Beutler and Alexander
Poltorak, Ph.D., several years ago. Double-stranded RNA signals
via TLR3—another member of the family. For this reason,
Beutler and his coworkers guessed that the mutation might
affect a molecule required for both TLR3 and TLR4 to signal
properly. They mapped the Lps2 mutation to a 216,000-base
pair region of chromosome 17. Of the eight genes in that region,
one gene, then called Trif, was a prime candidate because
it encoded an adaptor "TIR domain" protein—just the type
of protein that might participate in signaling from toll-like
receptors.
Beutler and his colleagues sequenced all of the genes in
this region and found a mutation affecting a single nucleotide
in the TrifLps2
gene. The mutation is a "frameshift" error—the 24 amino
acids at the tail end of the gene are exchanged for a completely
different set, and when the protein is translated in the cell,
it cannot function.
The fact that macrophages with these malfunctioning Trif
proteins did not respond to LPS suggests that Trif might make
a good target for treating sepsis, which can occur during
a widespread bacterial infection. During such an infection,
macrophages produce inflammatory chemicals, which help to
kill the bacterial cells. But if the systemic endotoxin levels
are too high, the macrophages respond by producing a lethal
amount of inflammatory chemicals.
Having a way to stop this would be a boon, because the current
prognosis for sepsis is dire. It can affect many parts of
the body, including the liver, kidneys, heart, intestines,
adrenal glands and brain, and death due to septic shock can
occur in a matter of hours. According to the Centers for Disease
Control and Prevention, sepsis is one of the ten leading causes
of both infant and adult mortality in the United States, and,
in 1999, directly caused more than 30,000 deaths.
Another interesting conclusion found in the paper is that
macrophages with no Trif protein can be divided into two different
populations—one pool of cells that are slightly responsive
to LPS, and one pool that are unresponsive. This is important
because scientists have long considered macrophages to be
a homogeneous population of cells.
After observing this, Beutler and his colleagues discovered
that even normal macrophages fall into different pools that
can be distinguished on the basis of how well they respond
to certain stimuli. The apparent heterogeneity might suggest
that macrophages specialize somewhat in their function.
"I would guess that some macrophages are better at killing
virus-infected cells than at coping with bacteria [and vice-versa],"
says Beutler.
The article, "Identification of Lps2 as a key transducer
of MyD88-independent TIR signaling" was authored by Kasper
Hoebe, Xin Du, Philippe Georgel, Edith Janssen, Koichi Tabeta,
Sung Ouk Kim, Jason Goode, Pei Lin, Navjiwan Mann, Suzanne
Mudd, Karine Crozat, Sosathya Sovath, Jiahuai Han, and Bruce
Beutler and appears in the Advance Online Publication feature
of the journal Nature on July 20, 2003. See: http://www.nature.com/nature/.
The article will appear in print later this year.
The work was funded by grants from the National Institutes
of Health, including a multi-center grant from The National
Institute of Allergy and Infectious Diseases (NIAID) that
has permitted TSRI investigators to study a broad range of
problems in innate immunity. Beutler and colleagues hope that
this work will be the first of many important discoveries
that will result from the approach of creating immune deficiencies
by introducing random mutations.

|

TSRI Professor Bruce Beutler studies
the innate immune system. His most recent paper appears in
an online version of the journal Nature
and will appear in print later this year.
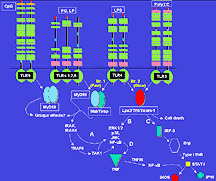
The Trif protein associates with different receptors
that detect a virus or a bacterium. Click
to enlarge.
|

