Programmable Antibodies—A Hybrid Cancer Therapy Described
by TSRI Scientists
By Jason Socrates
Bardi
Exploring the interface between organic chemistry and antibody
engineering, a team of scientists from the Department of Molecular
Biology and The Skaggs Institute for Chemical Biology at The
Scripps Research Institute (TSRI) has designed a "hybrid"
anticancer compound that physically combines the potent punch
of a cancer cell-targeting agent with the long-lasting dose
of an antibody.
Much as a hybrid bicycle is a cross between two bikes—a
road bike frame with mountain bike handlebars, for instance—this
hybrid compound is a cross between two molecules. One is a
traditional anticancer drug, a small molecule that targets
cancer tumors. The other is a type of antibody, which is a
protein produced in great abundance by the body's immune system
and found naturally in the bloodstream.
The hybrid of the two, described in an upcoming issue of
the journal Proceedings of the National Academy of Sciences,
was found to have a profound effect on the size of tumors
in mouse models—shrinking tumors of both Kaposi's sarcoma
and colon cancers in these preclinical studies. Moreover,
this approach is general enough that it could be used to design
hybrids against any number of cancers.
"A single antibody can become a whole multiplicity of therapeutics
simply by mixing it with the desired small molecule," says
TSRI Professor Carlos F. Barbas III, who is Janet and W. Keith
Kellogg II Chair in Molecular Biology.
Barbas and several other scientists at TSRI collaborated
in the interdisciplinary research, which one of them described
as existing at the interface of organic chemistry, biochemistry,
and immunology.
This team included Assistant Professor Christoph Rader,
Associate Professor and Skaggs Investigator Subhash Sinha,
postdoctoral fellow Mikhail Popkov, and TSRI President and
Skaggs Investigator Richard A. Lerner, who is Lita Annenberg
Hazen Professor of Immunochemistry and Cecil H. and Ida M.
Green Chair in Chemistry.
"The beauty of this [approach] is its generic design," says
Rader. "You have one antibody molecule and you can blend it
with the whole diversity of the organic chemistry world."
Steering and Support, Joined at the Hip
The TSRI team built the hybrid molecule with a "catalytic"
antibody, a small drug molecule, and a linker molecule that
joins the two. The hybrid thus formed borrows the wheels and
the frame of the antibody for supports and the handlebars
of the small drug molecule for steering ability.
Also called immunoglobulins, antibodies are proteins produced
by immune cells that are designed to recognize a wide range
of foreign pathogens. After a bacterium, virus, or other pathogen
enters the bloodstream, antibodies target antigens—proteins,
carbohydrate molecules, and other pieces of the pathogen—specific
to that foreign invader. These antibodies then alert the immune
system to the presence of the invaders and attract lethal
"effector" immune cells to the site of infection.
Antibodies have for many years been seen as useful therapeutics
for a number of human diseases ranging from rheumatoid arthritis
to leukemia because they are designed to target particular
cells and attract other parts of the immune system to the
site. There are a dozen antibodies that are approved as therapeutics
by the U.S. Food and Drug Administration, and many more under
development.
The hybrid the TSRI team created does not use the antibody's
targeting ability but rather its other properties—namely
its ability to stay around in the bloodstream. While many
small-molecule drugs are cleared from the blood by the kidneys
in a matter of minutes or hours, the large, soluble antibody
molecules are designed by the body to remain in the bloodstream
for long periods of time. In fact, in their experiments, Barbas
and his colleagues observed that their hybrid antibodies remained
in circulation for a week, while the small-molecule drug was
cleared in minutes.
Barbas and his colleagues used a catalytic antibody, since
these have the ability to react with other molecules like
a catalytic enzyme. In particular, the antibody they used
has a lysine residue at a key location. This lysine residue
allowed them to react the antibody with the small drug molecule
and "covalently" attach the two with a diketone linker.
"The diketone reacts with the reactive lysine residues in
the binding sites of the aldolase monoclonal antibody 38C2,
that we used, and you get the [hybrid] molecule," says Sinha.
This was more difficult than it sounds, however, since the
small molecule also had to be linked to the diketone without
disturbing the binding of the molecule to its receptor and
at the same time the diketone also reacts with the antibody.
"We had to build [from scratch] a molecule that we could link,"
says Sinha, who produced such a molecule in a 13-step organic
synthesis, starting with the chemical 4-bromo-3-methyl anisole.
Circulating and Guiding
The beauty of the hybrid is that while the antibody portion
keeps the hybrids circulating, the small-molecule portion
guides them towards cancer cells. In this case, the small
molecule they used guided the hybrids to target two molecules
known as the integrins alpha(v)beta(3) and alpha(v)beta(5).
Cancerous cells activate endothelial cells to express integrins
like alpha(v)beta(3) and alpha(v)beta(5) to promote the process
of angiogenesis, the formation of new blood vessels that bring
necessary nutrients and oxygen to hungry tumor cells. Block
angiogenesis, the thinking goes, and you can starve a tumor—like
drying out a lake by diverting all its tributaries. Many cancer
cells like breast, ovarian and prostate cancer also express
these integrins on their surface, providing for a potential
double-strike against the tumor itself as well as its key
blood supply.
In its study, the TSRI team found that the affinity of the
small molecule for the alpha(v)beta(3) and alpha(v)beta(5)
on the surfaces of the tumor cells steered the hybrids towards
the tumors. And once there, the antibody part of the hybrid
would activate other parts of the immune system—like
macrophages and the "complement" system—that recognize
the antibody and destroy the cells to which they are attached.
This proved to work well in the pre-clinical studies performed
by the TSRI team. In addition, the use of the targeting molecule
allowed the researchers to avoid one common difficulty with
developing antibody therapeutics—monoclonal mouse antibodies
don't normally target mouse antigens, which makes doing preclinical
studies tricky.
Moreover, say the authors, this hybrid approach could be
used as a broad drug-design strategy to rescue compounds that
are able to kill cancerous cells in the test tube but have
proven ineffective in human trials because they have a very
short half-life in the bloodstream. Alternatively, the technique
could provide killing function to drugs that may only bind
the tumor cells.
"There is a whole world of small molecules that have been
developed and tested in the clinic but have failed because
of low half-life or poor efficacy," says Barbas. "A single
antibody can be used [as a vehicle for many of these small
molecules]."
"In essence," says Popkov, "we have replaced the antibody
diversity with a chemical diversity. We can use this single
antibody as a template to recognize all the other molecules
[we desire]."
The article, "Chemically programmed monoclonal antibodies
for cancer therapy: Adaptor immunotherapy based on a covalent
antibody catalyst," authored by Christoph Rader, Subhash C.
Sinha, Mikhail Popkov, Richard A. Lerner, and Carlos F. Barbas,
III, is available online at: http://www.pnas.org/cgi/content/abstract/0931308100v1
and will be published in an upcoming issue of the journal
Proceedings of the National Academy of Sciences.
This work was supported by funds from The Skaggs Institute
for Research and an Investigator Award from the Cancer Research
Institute.

|

A team of TSRI scientists, including
(left to right) Mikhail Popkov, Christoph Rader, and Subhash
C. Sinha, explored the interface between organic chemistry
and antibody engineering in a new study. On the team but not
pictured are Carlos Barbas III and Richard A. Lerner. Photo
by Jason S. Bardi.
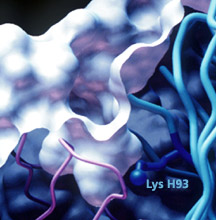
A targeting module derivatized with
a 1,3-diketone linker can program the specificity of an aldolase
antibody through reaction with its reactive lysine residue.
As shown in this crystal structure obtained in Ian A. Wilson's
laboratory, the reactive lysine residue is deeply buried,
yet accessible at the base of a hydrophobic pocket in the
antibody binding site.
|

