|
(Page 2 of 2)
Host Factors in Immunopathology
Strangely, aside from secondary infections, measles can
cause a variety of effects in a patient—called immunopathologies—and
determining the molecular causes of these is one area that
interests Oldstone greatly.
Measles virus most often causes an acute infection accompanied
by fevers and characteristic rashes. Frequently, though, it
also causes a subacute infection characterized by severe brain
disorders. And in some rare instances, measles causes a chronic,
progressive disease.
This is one of the great mysteries of viral infections—how
they can be so very different for different people. "How can
the same virus cause three phenotypes?" asks Oldstone.
Oldstone is able to address this question experimentally.
Several years ago, using transgenic techniques he developed
the first in vivo models that carry the measles receptors
and are susceptible to the virus. This allowed him and his
laboratory to replicate a significant part of measles infection
and learn about it.
The answer to the question of divergent phenotypes seems
to lie in the field of both host genetics and viral immunopathology,
in other words in the constellation of host factors and the
way in which the virus interacts with them. These interactions
determine the severity of the infection. The great majority
of symptoms that occur during an acute infection are due to
the reaction of the immune system.
"When you have an acute infection, the immune system is
designed to terminate it," says Oldstone. "Either you get
immunity or you get death."
But this depends on how widespread the infection is and
where it takes place. If it takes place in the brain or the
heart, the damage from the immune reaction might be so severe
that the organism cannot recover. However, an infection that
is localized in the skin might not be so severe.
Knowing what is turned on and off in the host organism and
how these molecular switches can be switched back to normal
is a major goal of Oldstone's research for another reason
as well—it illuminates how viruses are able to persist
in a host for years.
The Persistence of Viral Infections
In viral infections, a battle rages in the body. Sometimes
this battle is won by the host, sometimes it is won by the
virus, sometimes the host and the virus fight each other for
years in a protracted war of attrition, and sometimes they
coexist with remarkably little injury.
When a virus persists, the animal that is infected with
the virus lives a natural life but may have specific dysfunctions.
If the virus lives in the nerve cells, for instance, the learning
functions of the animal can be impaired. If the virus replicates
in the immune cells, the functioning of the immune system
can be impaired.
"For a virus to persist, it has to evade the host immune
system," says Oldstone.
Viruses often distort the function of cells, but in subtle
ways. For instance, the GAP-43 gene, which is involved in
cognitive function, can be turned off by lymphocytic choriomeningitis—a
virus that Oldstone has studied for a number of years. The
same virus can turn off the transcription factor that regulates
the expression of growth hormone, which results in stunted
growth.
"You don't see any distortion in the cells if you look at
them under a microscope," says Oldstone. "The brain and pituitary
gland look normal."
He looks at the mechanism, asking how the virus interacts
with the host and how the host interacts with the virus. He
also asks if this can be prevented.
Interestingly, when Oldstone began studying viruses, the
prevalent theory at the time was that when viruses persisted,
they did so because the host organism mounted no host response
at all to the infection. In fact, Oldstone determined that
this was not the case in the viral infections he looked at.
"I found that the host DID make an immune response," he
says, "but the immune response was not enough to clear the
infection."
And Speaking of History...
A few years ago, Oldstone wrote what might be considered
an unusual book for an academic scientist. Unusual, that is,
unless one considers the great tradition of literary scientists—Loren
Eisley, Carl Sagan, and Stephen Jay Gould to name a few—who
are both accomplished scientists and writers.
Perhaps as readers, we like to read scientist writers because
their knowledge of the field is profound, and we trust them
to guide us into the material—because we want to read
about science, whether reflections on paleontology or evolution,
or the galaxies, or time and space itself. We want to understand
and we look to scientist writers who have an immediate intimacy
with the subject matter.
And fascinating subject matter it is.
Viruses, Plagues, & History (Oxford, 1998) reads
like a cross between Albert Camus and Steven Ambrose—a
history wrapped in a narrative focused on a viral immunobiologist's
history of viruses interacting with our immune system through
time. His familiarity with the subjects allows him to move
seamlessly from discussing 100-year-old measles outbreaks
in Fiji to describing the immunopathology of the virus.
With chapters on smallpox, yellow fever, measles, polio,
ebola, HIV, influenza, and other subjects, Viruses
has a lot of material packed into its 230 or so pages. Still
in its first edition, the book has recently been released
in paperback and has been translated into several foreign
languages, including Spanish, Polish, Chinese, Japanese, and
even Hungarian.
"I've gotten invitations to speak about the book in several
museums and colleges," says Oldstone. These include the Art
and Science Museum in San Francisco and library and history
departments of Ohio State, Montana, and Augustona College.
What led him to undertake such a huge effort late in his
career? Like many of his contemporaries and many students
of virology, medicine, and public health who would follow,
Oldstone was inspired by Paul de Kruif's 1926 classic Microbe
Hunters. And he had other ulterior motives as well.
"I thought it might be fun to entice undergraduates and
graduates into the field," he says. "That's why I wrote the
book."
1 | 2 |

|
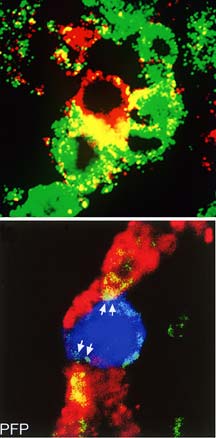
The top panel shows a single lymphocytic
chorlomeningitis virus (LCMV)-specific CD8 T killer cell engaging
three virus-infected cells in vivo in the brain. The bottom
panel shows a single LCMV CD8 T killer cell that has deposited
the chemical perforin on two virus-infected cells in the brain.
Deposition of perforin is required to lyse the virus-infected
cells and thus destroy them. (Data
from studies by Dorian McGavern and M.B.A. Oldstone.)
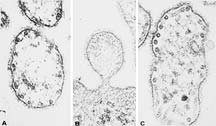
An electron micrograph showing measles
particles magnified 120,000 times. Micrography
from studies by M.B.A. Oldstone and Peter W. Lambert.
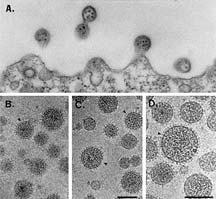
An electron micrograph showing lymphocytic
choriomeningitis virus (LCMV) virions. Micrography
from studies by M.B.A. Oldstone, Peter W. Lambert, and Michael
Buchmeier.
|

