

Scientists Describe Smoker's Enzyme
By Jason Socrates Bardi
A team of scientists at The Scripps Research Institute has solved the structure of a human molecule called CYP2A6, which is the principal enzyme in the body that degrades nicotine.
CYP2A6 is a protein that can be found in the endoplasmic reticulum of cells in the liver, where it is but one of a gauntlet of enzymes responsible for removing toxic chemicals from the body. In the case of nicotine, though, CYP2A6 almost single-handedly breaks down the chemical in the bloodstream as it circulates through the liver.
The recently solved structure reveals in fine detail the exact active site shape of the enzyme. Having the structural details makes it more feasible for researchers to design an inhibitor—somewhat the way that a clothing designer could fashion a great outfit for an individual given the person's exact measurements.
Because of its singular importance for metabolizing nicotine, blocking this enzyme would decrease the craving felt by a smoker, as less nicotine goes further. This could help smokers trying to quit through patches, gums, or any other standard nicotine replacement therapy by reducing replacement nicotine needs to very low doses.
"You get another advantage by inhibiting it," says Scripps Research Professor Eric F. Johnson. "[The enzyme CYP2A6] activates some of the carcinogens in tobacco smoke, and by inhibiting it, you can prevent the production of these carcinogenic metabolites."
Johnson conducted the study, which will appear in next month's issue of the journal Nature Structural & Molecular Biology, with Scripps Research colleagues, including Professor C. David Stout and former Research Associate Jason K. Yano.
Nicotine and its Removal
Nicotine is one of thousands of chemical components of cigarette smoke, and it is the main ingredient in tobacco that leads to addiction. This nicotine addiction fuels cigarette smoking, which is a major health problem in the United States. The latest report of the U.S. Surgeon General on the health effects of smoking calls smoking the leading preventable cause of death in American society. And, according to the U.S. Centers for Disease Control and Prevention (CDC), one in five deaths in the United States is related to smoking, and more than 400,000 Americans die each year from it—through cancers, cardiovascular diseases, and respiratory diseases.
When a person smokes, nicotine enters the bloodstream through the lungs. From there, it circulates through the vasculature until it reaches the liver. There, nicotine and many other exogenous chemicals that enter the bloodstream are metabolized or broken down by specific enzymes known as P450s. P450 enzymes break down these molecules by using the reactivity of an oxygen-binding molecule called a heme.
Scientists once thought that there was only one type of P450 enzyme, but in fact humans and other creatures have dozens or more. Humans in fact have at least 57 of these enzymes expressed throughout the body—particularly in tissues and membranes where toxins and other foreign compounds might enter. Many of them are broadly reactive, and together the various types of P450 enzymes have the capacity to recognize a broad spectrum of substances. Each has its own distinct active site with a distinct shape that allows it to recognize a certain type of corresponding compound.
The type of P450 enzyme in the liver that degrades nicotine is called CYP2A6. "It's the principal enzyme in the body that degrades nicotine. It accounts for approximately 80 percent of nicotine metabolism," says Yano, adding that CP2A6 plays a key role in tobacco addiction because it is so crucial for degrading nicotine and determining the level of nicotine on the bloodstream.
With that in mind, Yano, Johnson, Stout, and their colleagues set out to solve the structure of the CYP2A6 protein with two different inhibitors bound to it. The results of what was two years of work appears in next month's Nature Structural & Molecular Biology, a high-resolution structure of CYP2A6 bound to two chemicals: coumarin, which is a substrate of the enzyme, and methoxsalen, which is a known inhibitor.
The Business End of the Smoker's Enzyme
Solving the structure of CYP2A6 was no small feat. Structures of a few other P450 enzymes have been solved in recent years, but in general this class of enzymes has not yielded its structural secrets to molecular biologists because the enzymes are membrane-bound proteins—which are notoriously difficult to work with. Yano, Johnson, Stout, and their colleagues got around this fact by clipping off the "sticky" end of the molecule that sits in the membrane. This allowed them to concentrate on the end of CYP2A6 that contains the heme—the "business end," as Johnson likes to say.
The structure reveals that the enzyme has a compact active site, unusual for a P450 enzyme—most of its cousins have large active sites that can bind a number of substrates. But CYP2A6's active site is surrounded by several phenylalanine amino acids, and these bulky groups essentially crowd out large substrates that might otherwise bind. As a result, the substrates that do bind in the active site of CYP2A6 are small aromatic compounds, like nicotine. Moreover, unlike many P450 enzymes, CYP2A6 is only associated with the metabolism of a few different substrates. It does not make significant contributions to the oxidation of many drugs.
The structure's active site is also static, which means that it has the same shape regardless of which of different inhibitors are bound to it. The static nature of the structure makes it a good candidate for structure-based drug design because CYP2A6 inhibitors would not necessarily be widely cross-reactive with other enzymes involved in drug metabolism.
Inhibiting the CYP2A6 enzyme might be a viable way to help smokers kick the habit. Support for this hypothesis lies in the fact that people who are genetically deficient in the CYP2A6 enzyme (for example, a large number of people in Japan) tend to smoke fewer cigarettes overall and to quit smoking earlier in life.
Significantly, inhibiting the enzyme CYP2A6 might also be beneficial to a smoker's health by preventing CYP2A6 to metabolize nicotine into carcinogenic metabolites and activates other tobacco-related carcinogens to their active forms. Yano notes that the loss of the enzyme in the CYP2A6-deficient population seems to have a protective effect in this population, leading to a pronounced reduced risk of smoking-related cancer.
An inhibitor of CYP2A6 could also increase the effectiveness of nicotine replacement therapy—the patches and gums that have been one of the primary methods for achieving smoking cessation. Less nicotine would be needed to satisfy the craving, since the inhibited enzyme would not metabolize the nicotine as much. In addition, an inhibitor might allow oral nicotine replacement therapy—something that has been impossible thus far because nicotine that is taken orally must pass through the gut directly into the liver where a dense gauntlet of CYP2A6 molecules break much of it up. Taking an inhibitor of CYP2A6 along with a smaller dosage of nicotine contained in a pill might make the nicotine last longer in the bloodstream.
The details of the structures solved by the Scripps Research team have the potential to help in the development of therapeutic inhibitors of the enzyme.
The article, "Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen" by Jason K Yano, Mei-Hui Hsu, Keith J Griffin, C. David Stout, and Eric F Johnson appears in the September, 2005 issue of the journal Nature Structural & Molecular Biology. See: http://dx.doi.org/10.1038/nsmb971.
This work was supported by a grant from the National Institute of General Medical Sciences, a component of the National Institutes of Health, and through a fellowship provided to Jason Yano by California's Tobacco-Related Disease Research Program.
More information:
1) The Health Consequences of Smoking, a report of the U.S. Surgeon General: http://www.cdc.gov/tobacco/sgr/sgr_2004/index.htm
2) Cigarette Smoking-Related Mortality, statistics compiled by the CDC: http://www.cdc.gov/tobacco/research_data/health_consequences/mortali.htm
Send comments to: jasonb@scripps.edu

Professor Eric F. Johnson (left), former Research Associate Jason K. Yano, and their colleagues publish research in next month's issue of the journal Nature Structural & Molecular Biology that may lead to new help for smokers trying to kick the habit. Photo by Kevin Fung.
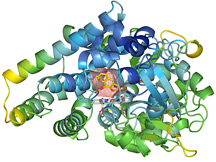
CYP2A6 with methoxsalen bound. Click to enlarge
