

Scientists Describe Antibody that Neutralizes Most HIV Strains
By Jason Socrates Bardi
A group of scientists from The Scripps Research Institute and several other institutions has solved the structure of a rare human antibody that broadly neutralizes human immunodeficiency virus (HIV), which causes acquired immunodeficiency syndrome (AIDS).
Neutralizing antibodies are soluble proteins secreted by adaptive immune cells into the bloodstream, following exposure to a virus. In the bloodstream, antibodies bind to viral particles in circulation, prevent them from infecting human cells, and lead to the viral particles’ destruction—thus neutralizing them.
Because neutralizing antibodies attack the virus before it enters cells, they can prevent HIV infection if they are present prior to exposure to the virus. An HIV vaccine would seek to elicit these neutralizing antibodies—just as existing vaccines against diseases such as measles, polio, hepatitis B, and hepatitis A elicit neutralizing antibodies against those viruses.
However, this is easier said than done. The body makes many antibodies against HIV, but they are almost always unable to neutralize the virus. Nonetheless, the immune systems of some patients with HIV have beaten the odds and have produced effective neutralizing antibodies. The structure of one of these, called 4E10, is described in the latest issue of the journal Immunity.
“This antibody is very broadly active,” says Scripps Research Professor Dennis Burton, who led the research with Scripps Research Professor Ian Wilson. “It neutralized nearly 100 different viral strains of HIV from all over the world. [During tests in the laboratory], every one of them was neutralized.”
4E10 was isolated from an HIV-positive individual about a decade ago by Burton and Wilson’s collaborator Hermann Katinger, a doctor at the Institute for Applied Microbiology of the University of Agriculture in Vienna, Austria, and one of the authors of the paper.
Significantly, the structure shows what an effective HIV-neutralizing antibody can look like. 4E10 targets an area on the HIV surface protein GP41 that the virus uses to fuse its membrane to the membrane of a human cell it is infecting. The target area is unusually close to the virus’s membrane surface, and the antibody has an unusual adaptation that might help it stick to the virus close to the membrane—a “finger” of amino acids with a propensity to dip down into the membrane and bring the antibody in contact with the target area.
Moreover, since the structure shows what the “epitope” looks like—the area on the HIV surface to which 4E10 binds—this work gives scientists insight into how to reverse-engineer a component of an HIV vaccine. The structure of this antibody could be used as a template to design an epitope mimic that would stimulate the human immune system to make 4E10 or similar broadly neutralizing antibodies against HIV.
“Once one knows what the epitope is, one can design mimics of it much more easily,” says Wilson, who is an investigator in The Skaggs Institute for Chemical Biology at The Scripps Research Institute.
The research article, "Broadly Neutralizing Anti-HIV Antibody 4E10 Recognizes a Helical Conformation of a Highly Conserved Fusion-Associated Motif in gp41: Implications for Vaccine Design” is authored by Rosa M. F. Cardoso, Michael B. Zwick, Robyn L. Stanfield, Renate Kunert, James M. Binley, Hermann Katinger, Dennis R. Burton, and Ian A. Wilson, and appears in the February, 2005 issue of the journal Immunity. The article can be accessed by journal subscribers at: http://www.immunity.com.
The research was supported by the National Institute of General Medical Sciences (NIGMS), the National Institute of Allergy and Infectious Diseases (NIAID), the International AIDS Vaccine Initiative (IAVI), The Skaggs Institute for Research, an American Foundation for AIDS Research fellowship, and the Elizabeth Glaser Pediatric AIDS Foundation. Drs. Burton and Wilson are members of IAVI’s Neutralizing Antibody Consortium, a consortium of scientists working together to design vaccines to elicit broadly neutralizing antibodies to HIV-1.
Send comments to: jasonb@scripps.edu
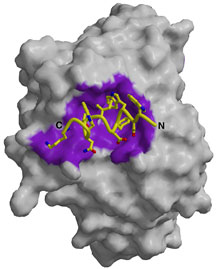
Antigen-binding site of anti-HIV antibody 4E10. Click to enlarge
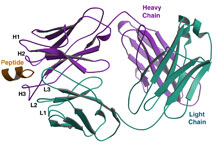
Overall view of the Fab 4E10-peptide epitope complex. Click to enlarge
