

Jet Lag—Not All In Your Head
By Jason Socrates Bardi
If you want to know what to blame when you get off an overseas flight and your body tells you it's the middle of the night even though the local time is 9:55 AM, you need look no further than your own liver.
The liver, like many tissues in humans, mice, mustard greens, and many other plants and animals, possesses an internal "circadian" clock that keeps track of time and coordinates its physiological and biochemical processes with the rhythm of the 24-hour cycle of day and night.
When we fly to another time zone, all of the clocks in all of our organs need to be adjusted—much like changing the time on a wristwatch upon landing. Only the liver doesn't respond as rapidly to changes in the sleep-wake cycle and is not so easy to reset, which leads to jet lag.
"Blame your liver for your jet lag," says Steve Kay, who is a professor of cell biology and the director of the Institute for Childhood and Neglected Diseases at The Scripps Research Institute.
In the last few years, Kay and his colleagues have been studying the different genes that control the timing of circadian rhythms of the various tissues. Not all of these genes are known, but it has become clear in the last few years that the mammalian clock is actually composed of many separate clocks that maintain different circadian rhythms specifically adapted to the various tissues of the body.
Coordinating the activities of all these different clocks is the job of the master circadian oscillator, or master clock, which in humans and other mammals is the suprachiasmatic nucleus, a tiny cluster of cells in the brain's hypothalamus with about 10,000 neurons. It sits above the optic chasm—the location where the optic nerves cross each other.
The question remained, however, of the relative importance of the master vs. local clocks. Did the timekeeping mechanisms in the body's periphery rely on the master clock to drive them? Or were they oscillating independently, relying on the master clock only for synchronization?
Now the Kay group has teamed up with a research group from Northwestern University and has reported, in a recent issue of the journal Current Biology, that individual fibroblast cellscontain independent clocks.
This observation was a first. While many experiments in the last several years had been designed to observe circadian rhythms in fibroblasts and other peripheral tissues, results showed that the level of expression of clock genes initially went up and down following a 24-hour cycle, but that the amplitude of the oscillation decreased progressively. After several days, the oscillating expression levels disappeared.
Suprachiasmatic nucleus neurons, on the other hand, displayed circadian rhythms measurable for many weeks, which has suggested to some scientists that the clocks in peripheral tissues such as fibroblast cells are not self-sustaining and that they might depend on the suprachiasmatic nucleus to drive them. However, the two sets of data were not necessarily comparable, as studies on fibroblast cells used groups of these cells, whereas the studies on the suprachiasmatic nucleus cells monitored individual neurons. There was no single cell data on peripheral cells.
In the new work, Scripps Research Visiting Professor David Welsh, M.D., Ph.D., Kay, and their colleagues used bioluminescence imaging to monitor circadian rhythms of clock gene expression in individual rat or mouse fibroblasts. In a recent issue of the journal Current Biology, the scientists report that they managed to monitor the individual fibroblast cells by using a supercooled, low-noise charge-coupled device (CCD) camera like those designed for astronomy—a sensitive device designed to collect light sources so dim that they emit fewer than a dozen photons a minute.
Using this camera, the researchers observed individual fibroblast cells to which had been added the luminescent luciferase gene so that they glowed ever so faintly whenever a particular clock gene called Per2 was expressed. Welsh, Kay, and their colleagues report that robust circadian rhythms persisted without damping for at least one to two weeks in these cells.
Welsh suggests the key to these results was observing individual cells rather than a group of them. The previous dampening effect may have occurred, says Welsh, because different cells keep slightly different cycles. The cells would be partially synchronized at the start of an experiment, but they would all be oscillating independently. Since some would have 24-hour rhythms, some 25-, and some 26-, the luminescence would drift out of phase after several days, leading to damping of the observable rhythm. Subtle differences in the chemical composition of the growth medium from place to place on the plate may have contributed to this effect.
To read the article, "Bioluminescence Imaging of Individual Fibroblasts Reveals Persistent, Independently Phased Circadian Rhythms of Clock Gene Expression" by David K. Welsh, Seung-Hee Yoo, Andrew C. Liu, Joseph S. Takahashi, and Steve A. Kay, see the December 29, 2004 issue of the journal Current Biology or go to: http://dx.doi.org/10.1016/j.cub.2004.11.057.
This work was supported by grants from the National Institute of Mental Health and by the Howard Hughes Medical Institute.
Send comments to: jasonb@scripps.edu

Professor Steve Kay leads a group that has been studying different genes that control the timing of daily biological rhythms.

Visiting Professor David Welsh is the lead author of the recent paper in Current Biology.
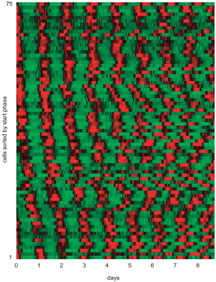
Primary fibroblast rhythms sorted by start phase. Image from Welsh et al. Courtesy of Current Biology. Click to enlarge.
