Mechanical Biology: Research on the "Leading Edge"
By Jason Socrates Bardi
"When I first beheld this apparition—for I could scarcely regard it as less—my wonder and my terror were extreme. But at length reflection came to my aid."
—Edgar Allen Poe, The Black Cat, 1843
Many topics—and words—to which a young college student is exposed are learned with difficulty and then in later life, happily forgotten.
But sometimes, these words come back to haunt those former students. For English majors, it may be topics like "parallel construction." For those who studied mathematics and physics, it is words like "deconvolution."
Deconvolution is the teasing apart of two things that are tightly coiled together. There are various numerical methods for performing this essential operation in data analysis, and there are many reasons to do so. For instance, sometimes two different signals collected by an instrument overlap and appear as one. Deconvoluting such signals is a reason to celebrate because it allows what may be two completely different phenomena to be studied separately.
Two scientists at The Scripps Research Institute recently joined intellectual forces to deconvolute an important biological machine associated with cell motion.
From Engineering to Biology and Back Again
Assistant Professor Gaudenz Danuser came to the Scripps Research Department of Cell Biology just over a year ago from the Swiss Federal Institute of Technology (ETH) in Zurich, where he was a junior faculty member in the Department of Mechanical and Process Engineering.
Danuser is an engineer by training. But several years ago, as a graduate student in electrical engineering and computer science at ETH studying topics like signal processing and robotics in instruments, he grew interested in cell biology.
"I became fascinated with crawling cells," he says. Danuser began to realize that the technologies and software he was developing might be most useful in biology. "If you think about what's happening in a cell, you [realize] that things are moving," he says. When movement is involved, he adds, you can begin to think about the mechanical forces that characterize and cause that movement—questions squarely in the realm of mechanics.
Danuser's new interests led him to a postdoctoral fellowship at Marine Biological Laboratory in Woods Hole, to a collaboration with Scripps Research Associate Professor Clare Waterman-Storer, and ultimately to his current position at Scripps Research.
Today, he does what he calls reverse systems engineering of dynamic cellular processes—analyzing how cells accomplish complicated feats like movement by applying a large framework of statistical processing to measurements of moving cells.
"I am a cell biologist with a different toolbox," he says.
Waterman-Storer, who has also been interested in the motion of cells for a number of years, has a more traditional background in molecular cell biology. See an earlier story: "Microscopy of Live Cells in Motion" (http://www.scripps.edu/newsandviews/e_20010423/waterman1.html)
The Importance of Cell Motility
Cell motility is of huge importance for a number of biological issues. In embryonic development, for instance, stem cells that will develop into nerve cells have to move to the correct location in the embryo before they can form nerve tissue and communicate with other cells.
Any time the skin is cut, the body will heal the wound by closing the skin and connecting the two sides of the cut. What drives this process is the action of cells that crawl forward to close and heal the wound. Once the two sides meet, the cells stop crawling and adhere to each other to reform solid tissue.
Likewise, motility is a vitally important area in cancer, since metastasis of cancerous cells is caused by cell motility. In fact, metastatic cells can be identified on plates by their ability to form colonies that continue to crawl and divide.
"If there were a way to selectively control tumor cell motility," says Waterman-Storer, "It could be used as an anti-metastatic therapeutic agent." Then cancers would remain local, forming tumors that could be easily excised.
Related to this is the topic of chromosome segregation—the event during the cell cycle in which duplicate copies of all the chromosomes inside a cell are separated so that a cell can then pinch apart and divide. This process must be efficient and accurate so DNA is not lost in the process.
"There is no manmade machine that has as high a reliability rate as the mitotic apparatus," says Danuser.
Imaging Dynamic Actin
One of the basic features of cell movement or motility is the treadmill-like effect of systems of molecular polymers called actin. Actin forms an extensive network of proteins at the leading edge of cells, and there it polymerizes (adds subunits) and depolymerizes (subtracts subunits).
This polymerization is what allows the cell to go forward. It generates a force that propels the cell membrane forward as the first "step" in movement. The actin polymers are cross-linked to each other so that they combine to form a meshwork of actin at the leading edge of the cell, and the addition of monomers at the ends of the actin polymers is what drives the meshwork forward.
"It's like a little treadmill all along the leading edge of the cell," says Waterman-Storer.
Biologists have been studying actin's role in cell motility for several decades, and this work has picked up in recent years, including at Scripps Research, where Danuser and Waterman-Storer are both members of the Center for Integrative Molecular Biosciences (CIMBio) on campus. CIMBio is a collaborative effort whose mission is to foster multidisciplinary studies of molecular machines, with the aim of determining their structure, their mechanism of action, and their dynamic behavior in the context of living cells and whole organisms.
Danuser and Waterman-Storer work on cell motility and migration, concentrating on how actin and other molecules play a role in making a cell protrude—the way that it will extend its leading edge. Scientists believe that most of the action that propels a cell forward takes place in this area. Danuser and Waterman-Storer have spent the last few years developing and validating a technology that allows measurements of this action to be made.
"We are trying to develop technologies that allow us to characterize cell dynamics under the light microscope with a completely new level of information content accuracy," says Danuser.
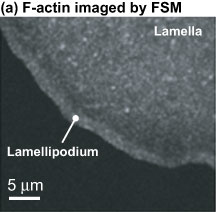
Using technology called fluorescent speckle microscopy, it is possible to image the difference between the leading edge of a cell and an area a micron back.
Fluorescent **Speckle** Microscopy
Fluorescent speckle microscopy as it exists today reflects the work of both Danuser's and Waterman-Storer's laboratories.
The technology is based on a method that Waterman-Storer and her former boss E.D. Salmon developed in the late 1990s while she was a postdoctoral fellow is his laboratory at the University of North Carolina at Chapel Hill.
In fluorescent microscopy technique, fluorophores (small molecules that absorb and reemit photons of a particular wavelength) are covalently attached to actin subunits and then microinjected into a cell. Scientists can then illuminate the cells with a colored light source and train a microscope camera to capture the reemitted photons.
But scientists had problems imaging actin and other parts of the cytoskeleton in living cells using traditional fluorescence microscopy because of the high concentration of fluorophores. Further complicating matters is the fact that actin filaments in cells form bundles and cross-linked meshes, making it impossible to see individual filaments. With traditional fluorescence microscopy, because the actin would be too evenly labeled with fluorescing molecules, watching actin motion is nearly impossible.
"It's like looking at a brick wall from a distance, where you cannot see the individual bricks and they are all red," Waterman-Storer says. "That wall could be moving in front of you, but if you can't resolve the individual bricks, you wouldn't know if the wall were moving or stationary."
Fluorescent speckle microscopy has provided a way to quantitatively analyze actin dynamics in vivo. By analogy, this technique resolves the movement of the wall by painting only certain bricks white, so that movement can be tracked. In fact, fluorescent speckle microscopy uses about 100 times less fluorescent material than fluorescent analog cytochemistry—only about one tenth of one percent of the subunits are labeled—but the lesser amount enables researchers to track how the whole moves.
The technology requires statistical analysis tools to detect and analyze the speckles. Danuser and Waterman-Storer have been developing these over the last few years to track these million points of light and interpret their kinetics—the rates of polymerization and depolymerization—and kinematics—the direction, and velocity of actin filament movement. Rigorous statistical analysis may also reveal properties that might not be obvious in a simple qualitative inspection of the data.
In his office a few months ago, Danuser showed a visitor a few movie clips of moving cells imaged with fluorescent speckle microscopy "just to give a feeling" of what he was talking about.
On the screen the actin networks at the front of a cell appear like a two-dimensional waterfall with an impossible flood of white speckles rushing away from the edge of the cell under the microscope as the cell creeps forward on the screen.
There may be a half million to a million speckled markers, Danuser comments. "All of them," he says, "are automatically tracked by a computer and analyzed [for their motion]."
Upon Further Analysis
Danuser and Waterman-Storer have a paper in this week's issue of the journal Science in which they analyze actin polymerization at the leading edges of a special type of cells called epithelial cells that can be found in places like the lungs of newts.
These cells, when undergoing wound healing, have a free edge that does not contact other cells, and they will crawl in that direction. This makes them good subjects to examine under the microscope.
In their report, the scientists describe how fluorescent speckle microscopy movie clips reveal how the actin polymerization at the leading edge of the epithelial cells propels those cells forward. The report is the first real application of their combined efforts to image cells in motion, and significantly, it gives a new physical description of the role actin plays in cell motility.
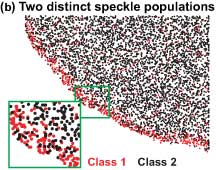
In their Science article, Danuser and Waterman-Storer show that the actin network that propels the cell forward is actually composed of two distinct, overlapping networks of actin polymers assembling at the leading edge of a cell.
The researchers generated maps of local actin polymerization and depolymerization rates. The maps showed, as one would expect, a strong burst of polymerization at the leading edge, but, unexpectedly, strong depolymerization afterwards, with about 90 percent of the actin depolymerizing within the first few microns.
When the researchers then classified speckles into two populations, depending on whether they were fast-moving and short-lived or slow-moving and long-lived, they found that these distinct classes of speckles showed differences in the organization of their polymerization and depolymerization patterns, which could be explained by two distinct but overlapping actin networks. The fast-moving short-lived network wiggles on top of another, slower-moving and with a differently organized polymerization activity—something Danuser likens to surfing.
The fast-moving network is the lamellipodium, which is at the very front of the moving cells. This depends, among others, on the protein Arp2/3 and assembles at the leading edge of cells and completely disassembles within a few microns, and these dynamics seem to drive the random protrusion and retraction of the edge of the cell.
The other, slow-flowing network is the lamella. It extends almost to the front of cells and obtrudes and contracts in cells. The lamellum is a somewhat random network of actin molecules undergoing polymerization and depolymerization and whose motion depends on the action of myosin.
The lamella pushes the lamellipodium forward and advances the moving cell by exerting forces locally that generate other tensile forces within the cell. These forces are carried through the cytoskeleton against sites of adhesion with the extracellular matrix.
Biologists have long believed that it is primarily this lamellipodium protrusion that allows a cell to move. But Danuser and Waterman-Storer discovered otherwise.
"In terms of the mechanics of making the cell move, [the lamellipodium is not important]," says Waterman-Storer.
To demonstrate this, they used chemicals that targeted one type of network, and discovered that when the fast-moving and short-lived lamellipodium network was removed, the cells moved faster.
What's Next
Cell biology is by its very nature complicated, and cellular phenomena in general involve multiple interacting proteins and molecular signals with complex biochemical output and control.
While Danuser and Waterman-Storer have found something new in a system that has been extensively studied for years, they admit that there is still much work to be done. Now that they have mapped the kinetics and motion of the two different networks, they plan to continue developing their sensitive technique to understand the signals and mechanical components of cells movement.
"We have to look for new signals and to understand the coordination of these two networks," says Danuser.
Still, Waterman-Storer is upbeat about the possibilities. "[Fluorescent speckle microscopy] is going to be important for understanding cell structures and processes in general," says Waterman-Storer. "We could use this to look at nuclear pores or focal adhesion structures. It's an exciting example of the sort of interdisciplinary science that Scripps is known for," she adds.
Send comments to: jasonb@scripps.edu